Atoms Elements and Ions The Language of Chemistry












































- Slides: 99

Atoms, Elements, and Ions

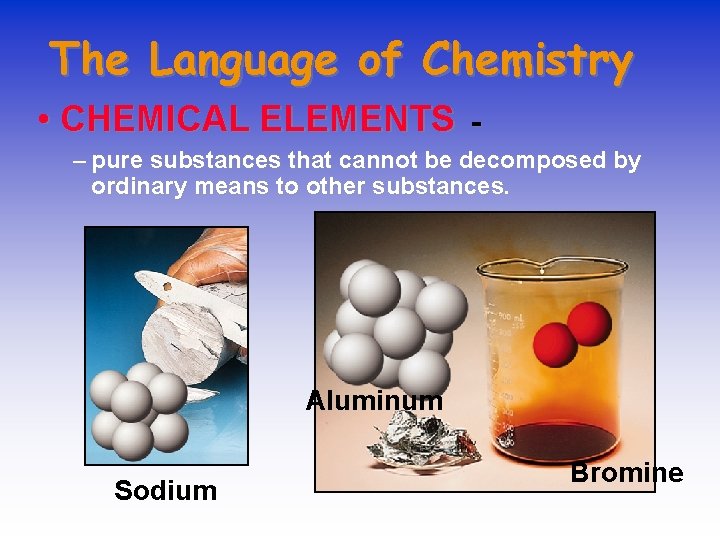
The Language of Chemistry • CHEMICAL ELEMENTS - – pure substances that cannot be decomposed by ordinary means to other substances. Aluminum Sodium Bromine

Lesson 1 The Atom: From Idea to Theory Essential Question • How has theory of the atom evolved over time? Objectives • To summarize Dalton’s atomic theory. • To explain the laws that support Dalton’s atomic theory.

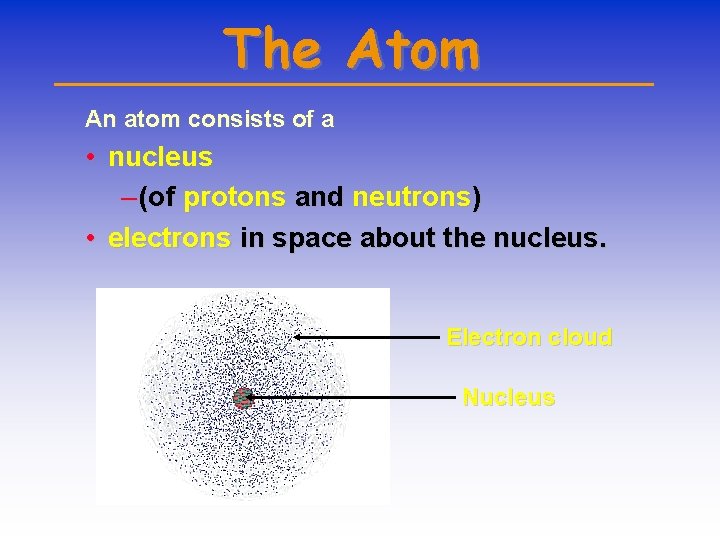
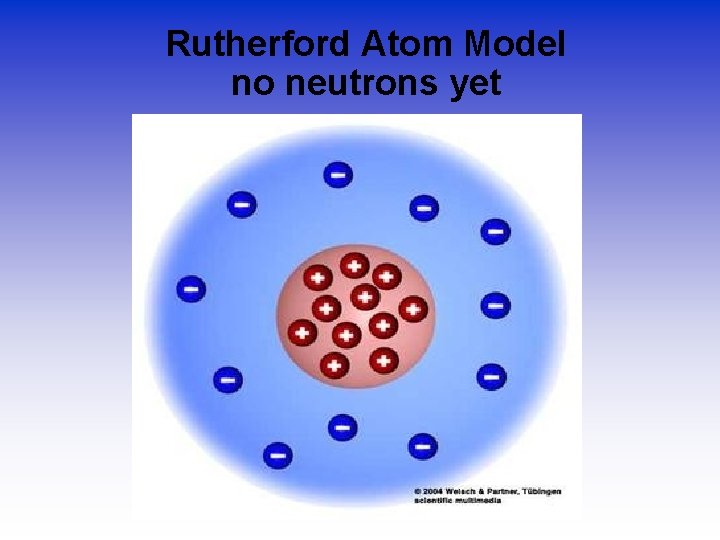
The Atom An atom consists of a • nucleus – (of protons and neutrons) • electrons in space about the nucleus. Electron cloud Nucleus

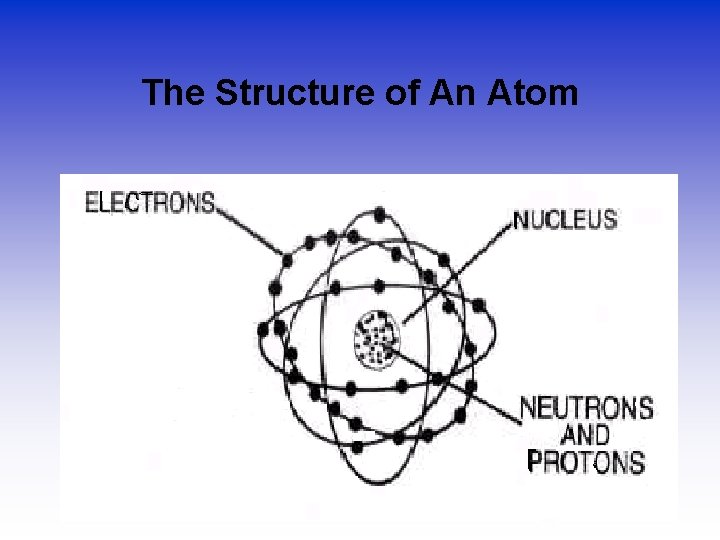
The Structure of An Atom

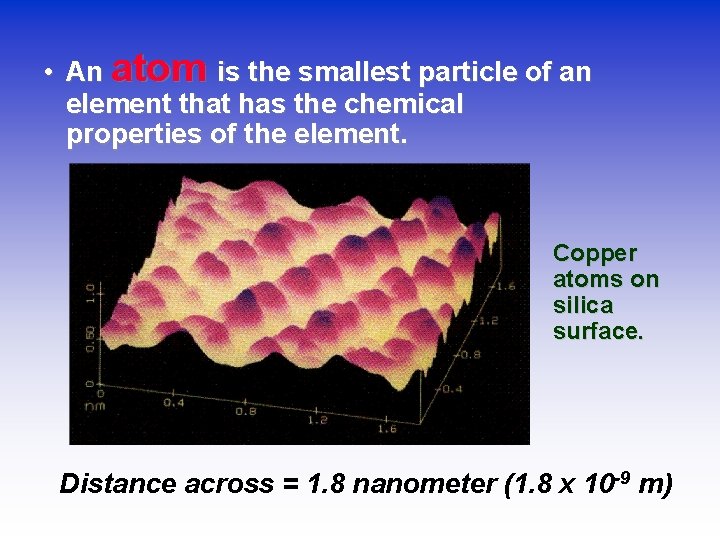
• An atom is the smallest particle of an element that has the chemical properties of the element. Copper atoms on silica surface. Distance across = 1. 8 nanometer (1. 8 x 10 -9 m)

History of the atom • Not the history of atom, but the idea of the atom. • Original idea began 1. Ancient Greece (400 B. C. ) » Democritus- Greek philosopher • “father of modern science” 2. India (600 B. C. ) 1. Hindu Nyaya philosophy Not really sure which one.

History of Atom • Looked at beach • Made of sand • Cut sand - smaller sand n. Smallest possible piece? n. Atomos - not to be cut

Another Greek • Aristotle - Famous natural philosopher • Believer of the 4 earthly elements – Fire - Hot – Air - light – Earth - cool, heavy – Water – wet • Plus one of his own » Aether – divine/heavenly bodies • Blend these elements in different proportions to get all substances

Who Was Right? • • Greek society was slave based. Beneath famous to work with hands. Did not experiment. Greeks settled disagreements by argument. Aristotle was more famous, so he won. His ideas carried through middle ages. Later, Alchemists attempted to change lead to gold.

Who’s Next? • Late 1700’s - John Dalton- from England. – Math & natural philosophy tutor- summarized results of his experiments and those of others. • Dalton’s Atomic Theory • Combined ideas of elements with that of atoms.

Dalton’s Atomic Theory 1. matter is composed, indivisible particles (atoms) 2. all atoms of a particular element are identical 3. different elements have different atoms 4. atoms combine in certain whole-number ratios 5. Chemical reactions involve the rearrangement of atoms. Ø No atoms are created, destroyed, or changed into atoms of any other elements.

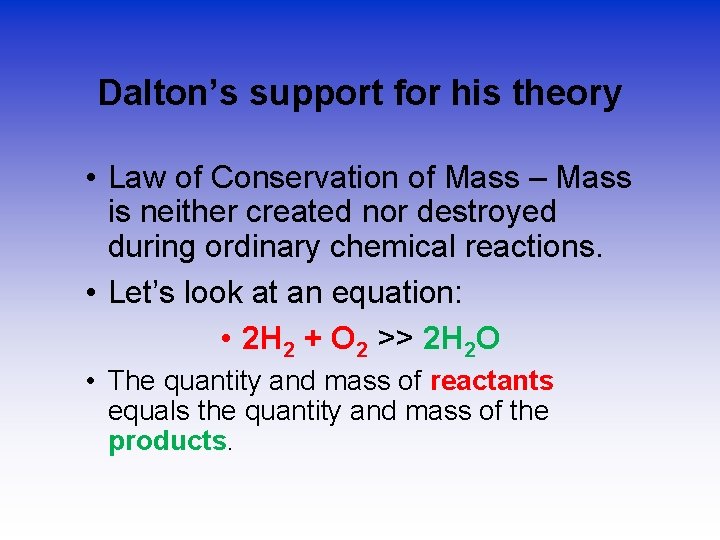
Dalton’s support for his theory • Law of Conservation of Mass – Mass is neither created nor destroyed during ordinary chemical reactions. • Let’s look at an equation: • 2 H 2 + O 2 >> 2 H 2 O • The quantity and mass of reactants equals the quantity and mass of the products.

Problems with Dalton’s Atomic Theory? 1. matter is composed, indivisible particles Atoms Can Be Divided, but only in a nuclear reaction 2. all atoms of a particular element are identical Does not Account for Isotopes (atoms of the same element but a different mass due to a different number of neutrons)! 3. different elements have different atoms YES! 4. atoms combine in certain whole-number ratios YES! Called the Law of Definite Proportions 5. In a chemical reaction, atoms are merely rearranged to form new compounds; they are not created, destroyed, or changed into atoms of any other elements. Yes, except for nuclear reactions that can change atoms of one element to a different element

Modern Atomic Theory • We know today that atoms are made of electrons, protons, neutrons. • We will study that next. Even More Modern Atomic Theory • Known as the Standard Model • protons & neutrons are made of six different “flavors” of quarks. • Electrons are made of leptons

Plus there’s more • Fermions Baryons • Neutrinos Gluons • Higgs-Boson – the most fundamental particle • Not to mention that EVERY particle of matter has an antimatter counterpart. • But we are not studying this stuff!

Learning Check 1 a 1. C + O 2 >> ? ? According to the Law of Conservation of Mass, the correct answer for the product is: A. B. C. D. CO C 2 O CO 2 C 1/2 O

Learning Check 1 b • Which of Dalton’s statement(s) is NO longer correct today? 1. All matter is composed of atoms. 2. Atoms of any given element are identical, and are different than atoms of another element. 3. Atoms cannot be created, destroyed, or subdivided. 4. Atoms of different elements combine in whole number ratios. 5. In chemical reactions, atoms are combined, separated, or rearranged. Isotopes-atoms of an element with different # neutrons Nuclear fission – splitting an atom

Learning Check 1 c • Explain how an idea, an opinion, a theory, and a law all become connected? • First, someone has an idea; then persuades others to believe, which is an opinion. Skeptical people need proof. Experiments and data provide proof which creates a theory. After many, many years of experiments trying to disprove without success, a theory becomes a law.

Lesson 2 Structure of the Atom Essential Question • How has scientific discovery and technology supported the structure of the atom? Objectives • To summarize the experiments that contributed to the structure of the atom. – Describe the structure of an atom including location of protons, electrons, and neutrons with respect to the nucleus. – Distinguish among protons, electrons, and neutrons in terms of relative mass and charge.

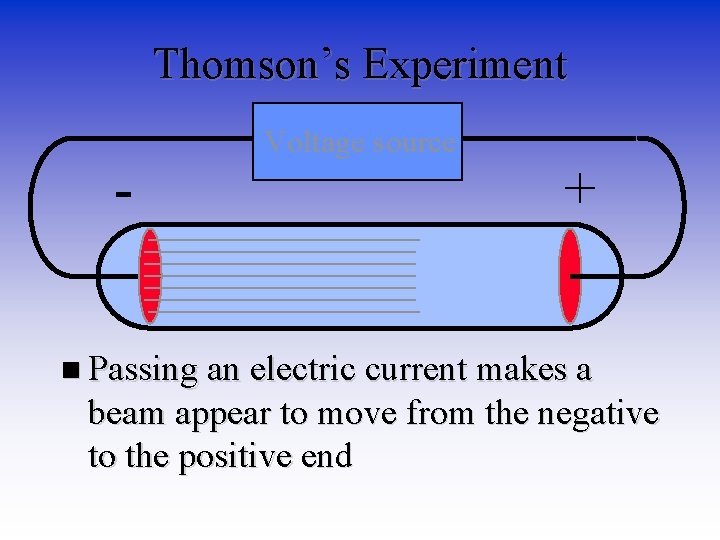
Parts of Atoms • J. J. Thomson - English physicist. (1897) • Made a piece of equipment called a cathode ray tube. • It is a vacuum tube - all the air has been pumped out.

Thomson’s Experiment - Voltage source Vacuum tube Metal Disks +

Thomson’s Experiment - Voltage source +

Thomson’s Experiment - Voltage source +

Thomson’s Experiment - Voltage source +

Thomson’s Experiment - Voltage source + n Passing an electric current makes a beam appear to move from the negative to the positive end

Thomson’s Experiment - Voltage source + n Passing an electric current makes a beam appear to move from the negative to the positive end

Thomson’s Experiment - Voltage source + n Passing an electric current makes a beam appear to move from the negative to the positive end

Thomson’s Experiment - Voltage source + n Passing an electric current makes a beam appear to move from the negative to the positive end

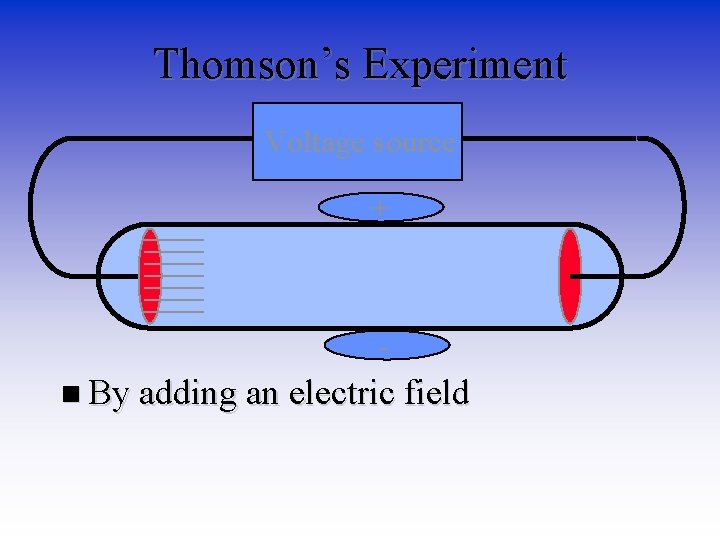
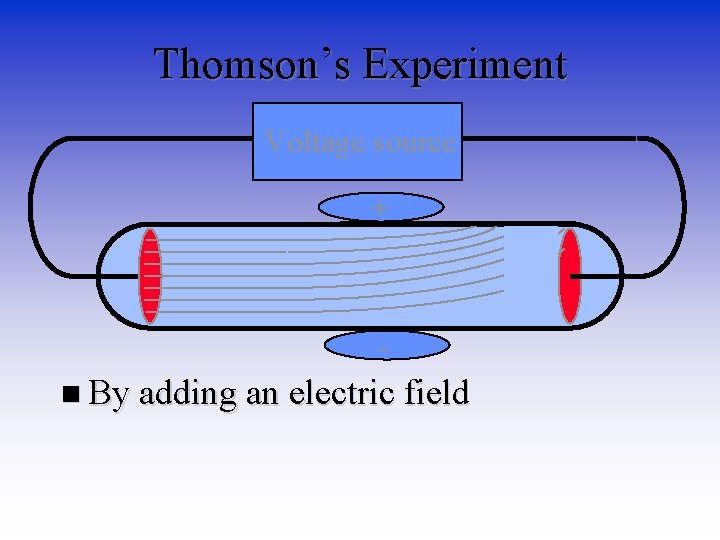
Thomson’s Experiment Voltage source • By adding an electric field

Thomson’s Experiment Voltage source + n By adding an electric field

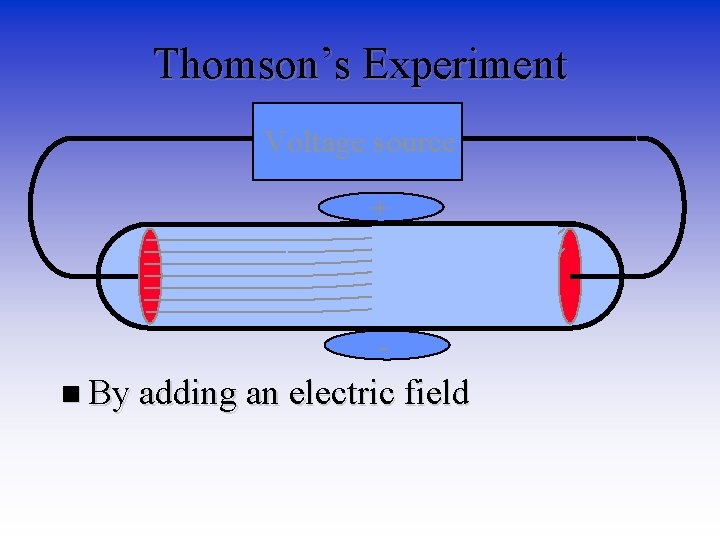
Thomson’s Experiment Voltage source + n By adding an electric field

Thomson’s Experiment Voltage source + n By adding an electric field

Thomson’s Experiment Voltage source + n By adding an electric field

Thomson’s Experiment Voltage source + n By adding an electric field

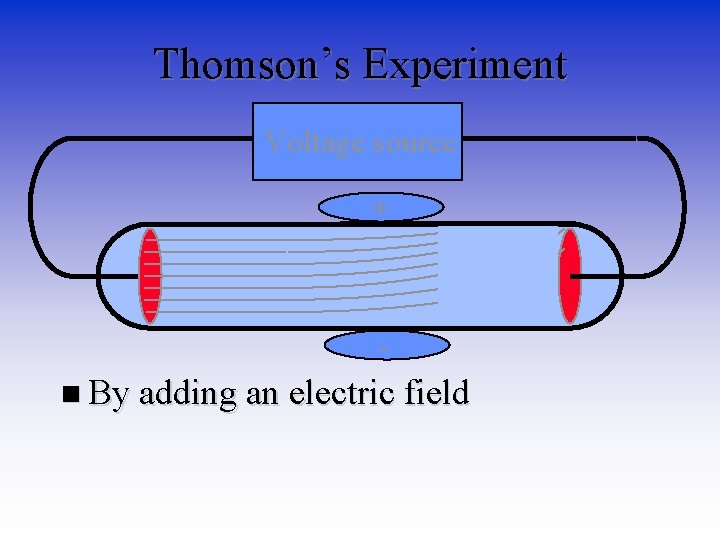
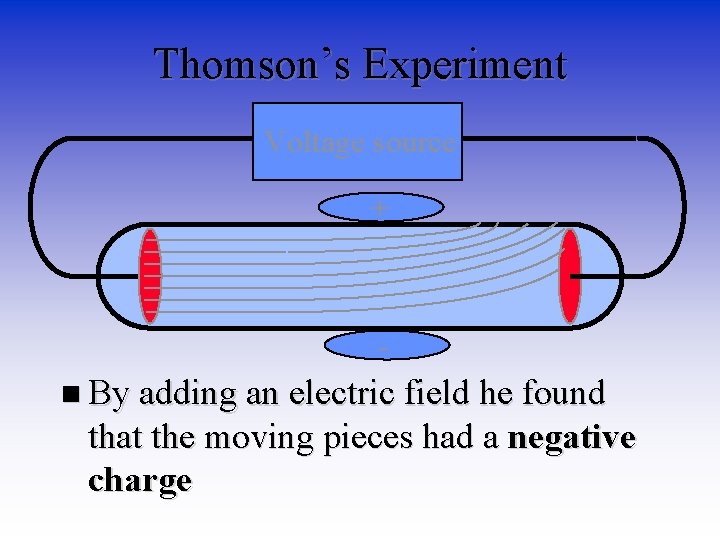
Thomson’s Experiment Voltage source + n By adding an electric field he found that the moving pieces had a negative charge

More from Thomson • JJ Thomson third experiment confirmed that the negative charge was coming from very tiny particles (1000 x smaller than hydrogen atom) and were not rays. He called them electrons. • In 1904 Thomson proposed his model of an atom. – Original knickname - Plum Pudding Model – Today’s knickname – Blueberry Muffin model (Why? )

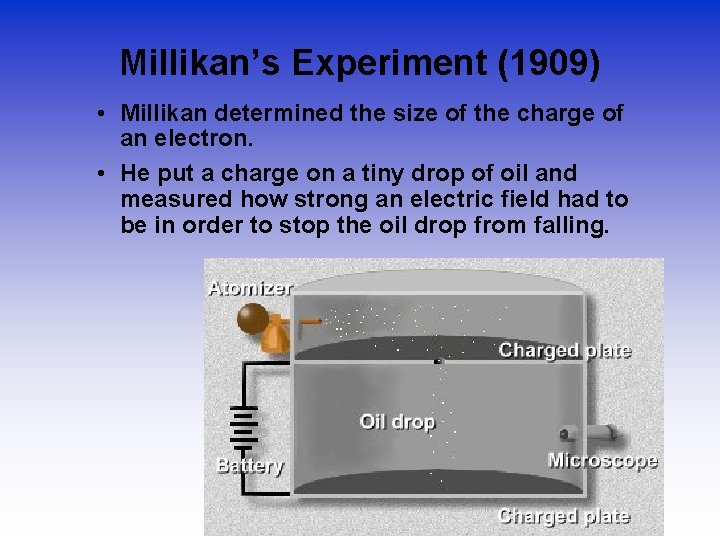
Millikan’s Experiment (1909) • Millikan determined the size of the charge of an electron. • He put a charge on a tiny drop of oil and measured how strong an electric field had to be in order to stop the oil drop from falling.

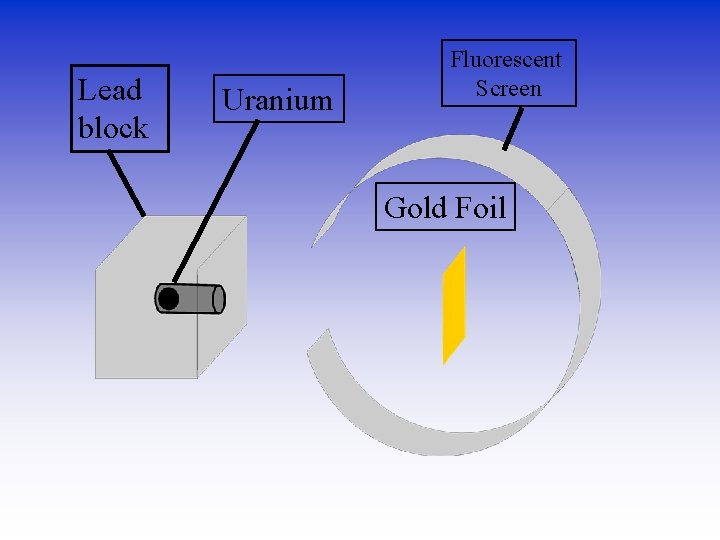
Rutherford’s experiment • Ernest Rutherford -English physicist. (1911) – Believed in Thomson’s model of the atom (1904). – Wanted to see how big they are. – Used radioactivity. – Alpha particles - positively charged pieces- helium atoms minus electrons – Shot them at gold foil which can be made a few atoms thick.

Rutherford’s experiment • When an alpha particle hits a fluorescent screen, it glows. • Here’s what it looked like

Lead block Uranium Fluorescent Screen Gold Foil

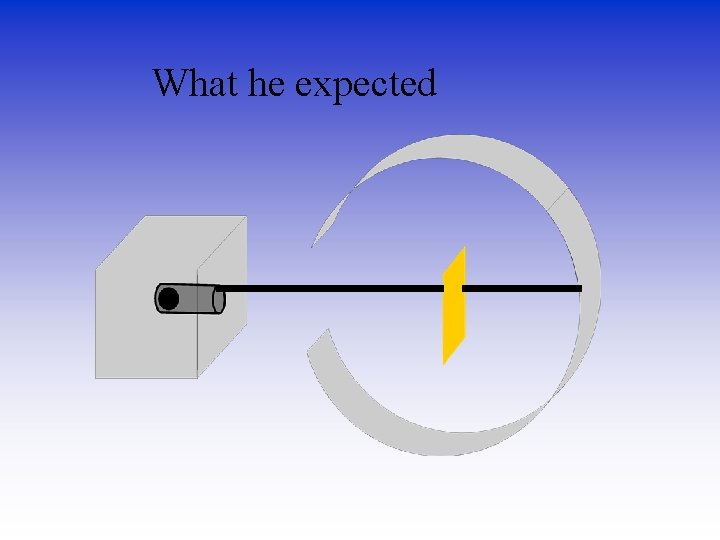
He Expected • The alpha particles to pass through without changing direction very much. • Because…? • …the positive charges were thought to be spread out evenly. Alone they were not enough to stop the alpha particles.

What he expected

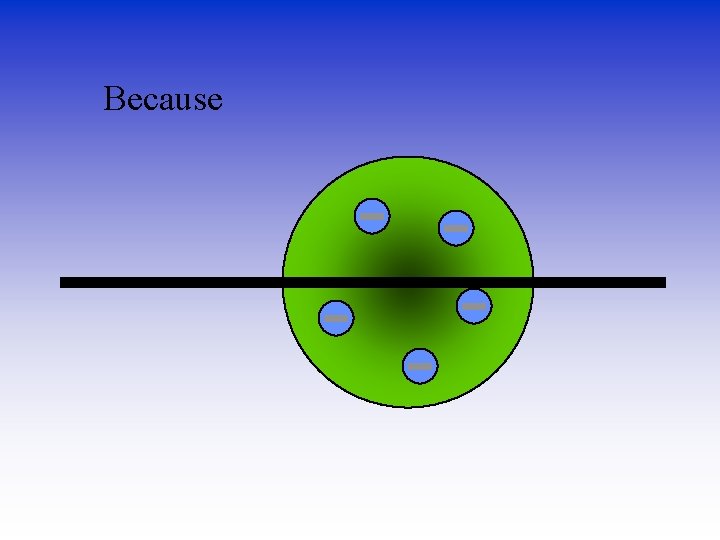
Because

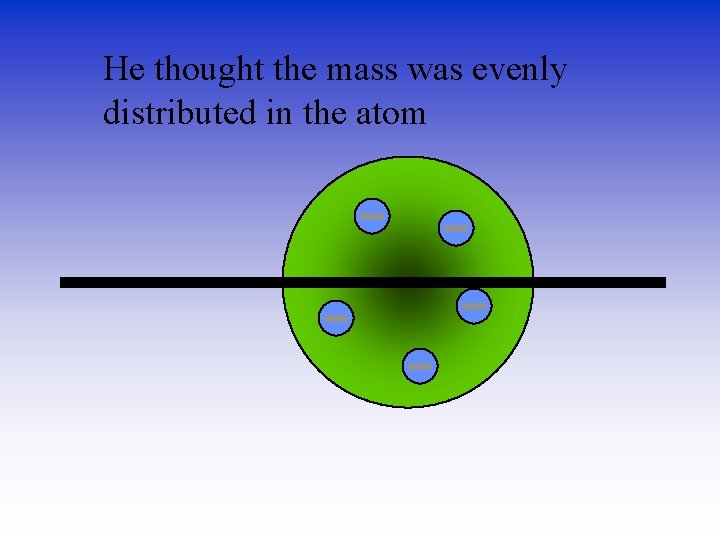
He thought the mass was evenly distributed in the atom

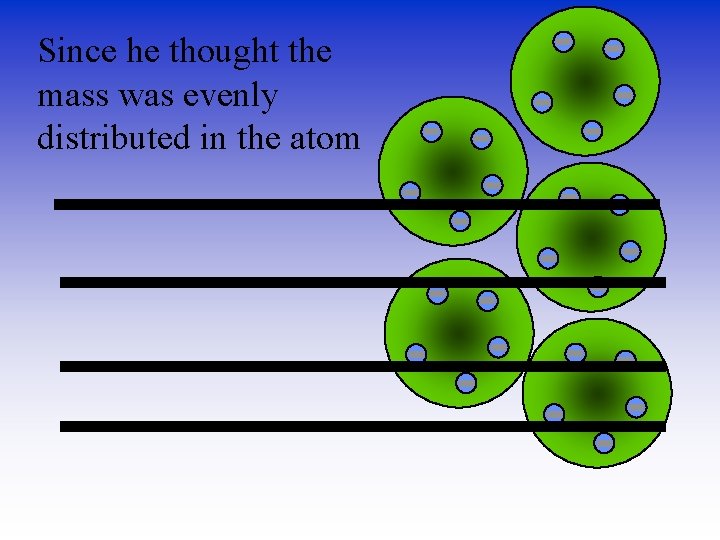
Since he thought the mass was evenly distributed in the atom

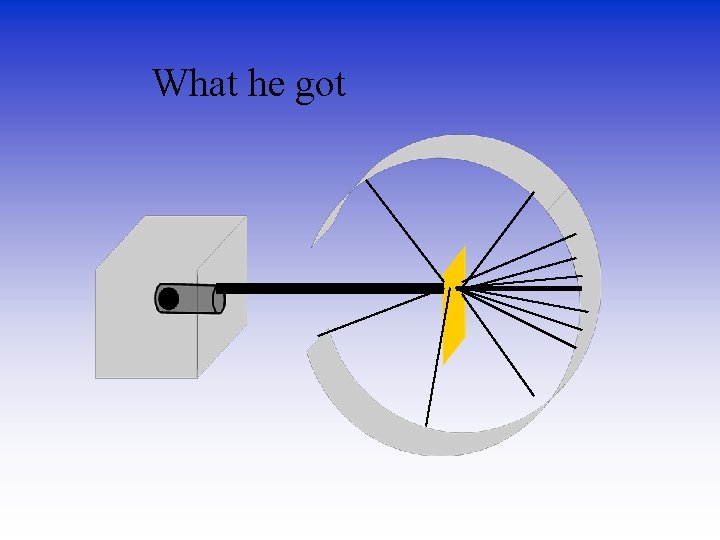
What he got

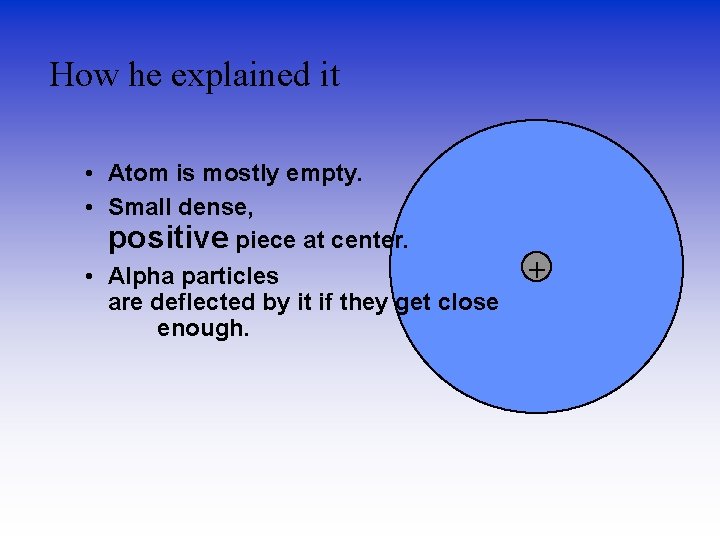
How he explained it • Atom is mostly empty. • Small dense, positive piece at center. • Alpha particles are deflected by it if they get close enough. +


Density & the Atom Conclusion • Since most of the particles went through, it was mostly empty space. • Because the pieces turned so much, the positive pieces were heavy. • Small volume, big mass, big density. • This small dense positive area is the nucleus.

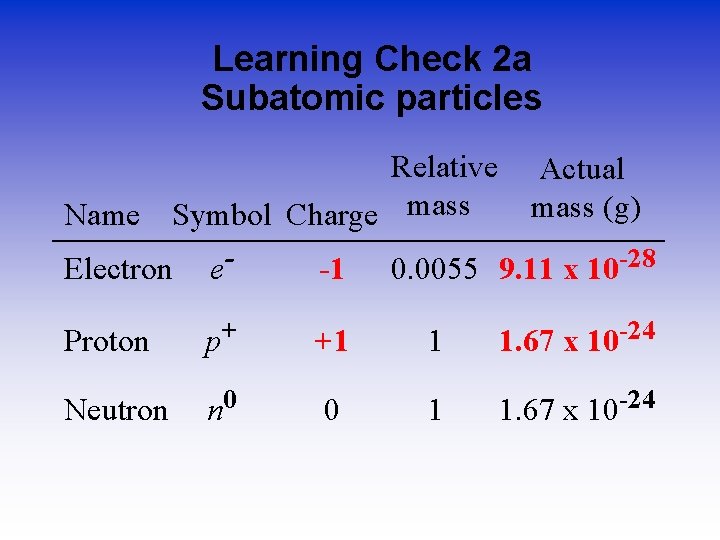
Learning Check 2 a Subatomic particles Relative Name Symbol Charge mass Actual mass (g) Electron e- -1 0. 0055 9. 11 x 10 -28 Proton p+ +1 1 1. 67 x 10 -24 Neutron n 0 0 1 1. 67 x 10 -24

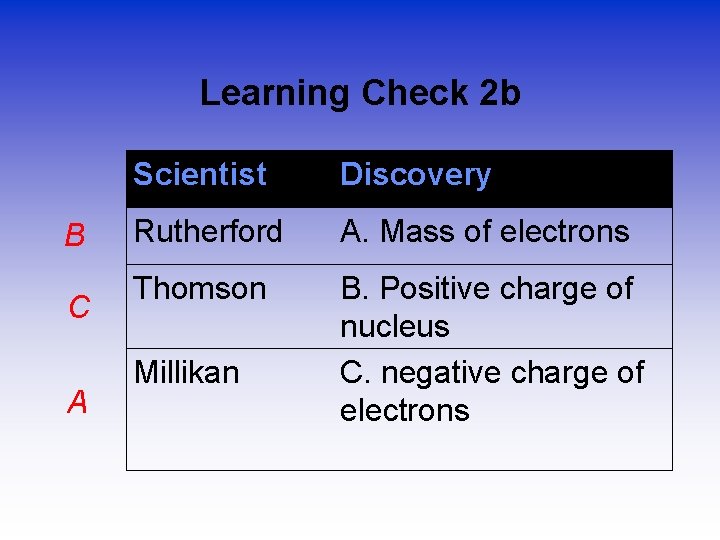
Learning Check 2 b B C A Scientist Discovery Rutherford A. Mass of electrons Thomson B. Positive charge of nucleus C. negative charge of electrons Millikan

More on Rutherford • 1911 – he named the center of atom nucleus, meaning “little nut. ” • Designed a model of the atom • 1920 – After further experiments Rutherford named the + charged particles in the nucleus – protons. • He predicted that there was a mass of neutral charge particles in the nucleus, but did not conduct any experiments.

Rutherford Atom Model no neutrons yet

Discovery of the neutron • It was known, but not understood, that the A was approximately twice the value of the Z for an element. • 1932 James Chadwick conducted experiments using radioactive material. • Discovered a particle in the nucleus with no charge – called it the neutron.

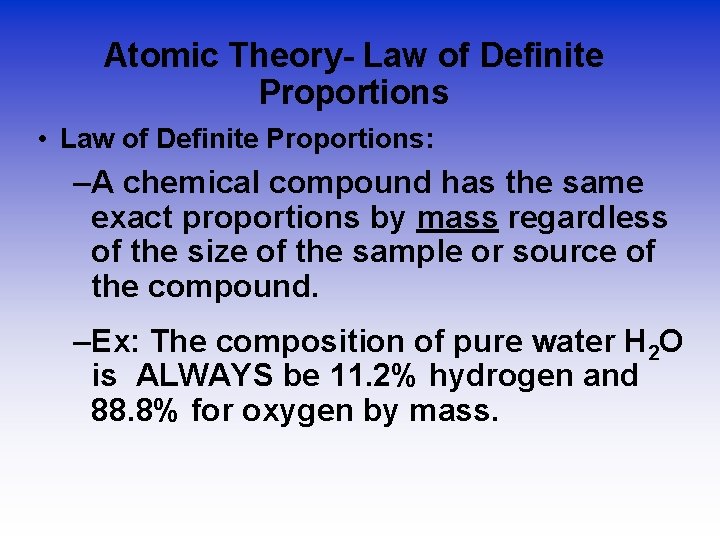
Atomic Theory- Law of Definite Proportions • Law of Definite Proportions: –A chemical compound has the same exact proportions by mass regardless of the size of the sample or source of the compound. –Ex: The composition of pure water H 2 O is ALWAYS be 11. 2% hydrogen and 88. 8% for oxygen by mass.

Example 1 •

Law of Definite Proportions Worksheet 1. Remember your Rules of Significant Figures 1. Multiply/Divide – Limiter is the value with least amount of sig figs. 2. Add/Subtract – Limiter is value with least amount of decimals. 2. When multiplying by 100%, the % sign is the unit of measure. Do not unit % key on calculator.

Example 2 • A sample of sodium chloride Na. Cl weighing 175. 35 g contains 60. 6% Cl by mass. 1. How many grams of Cl are in the sample? 2. How many grams of Na are in the sample?

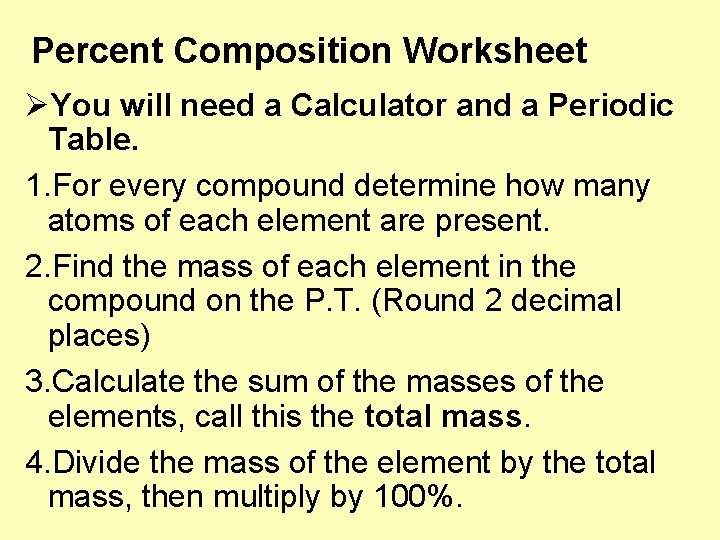
Percent Composition Worksheet ØYou will need a Calculator and a Periodic Table. 1. For every compound determine how many atoms of each element are present. 2. Find the mass of each element in the compound on the P. T. (Round 2 decimal places) 3. Calculate the sum of the masses of the elements, call this the total mass. 4. Divide the mass of the element by the total mass, then multiply by 100%.

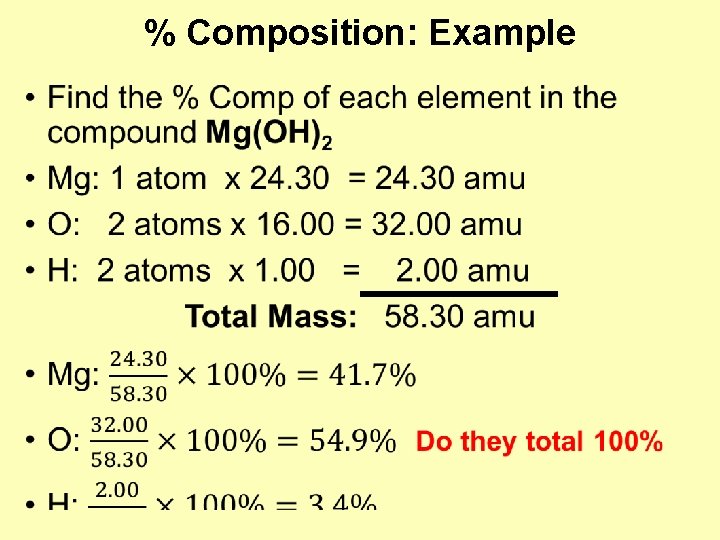
% Composition: Example •

Laws Pertaining to Atomic Theory. Law of Multiple Proportions • Law of Multiple Proportions – Is when 2 elements combine to form more than one compound. The ratio of the masses of the second element when combined with a certain mass of the first element is ALWAYS a ratio of the small whole numbers. – Ex: CO vs. CO 2. In CO 1. 33 g oxygen combine with 1 g carbon, while in CO 2, there are 2. 66 g of oxygen that combine with 1 g carbon.

Lesson 3 Counting Atoms Essential Question How do you distinguish between an atom, an ion, and an isotope? Objectives • To calculate the atomic number, mass number, and average atomic mass. • To explain what isotopes are.

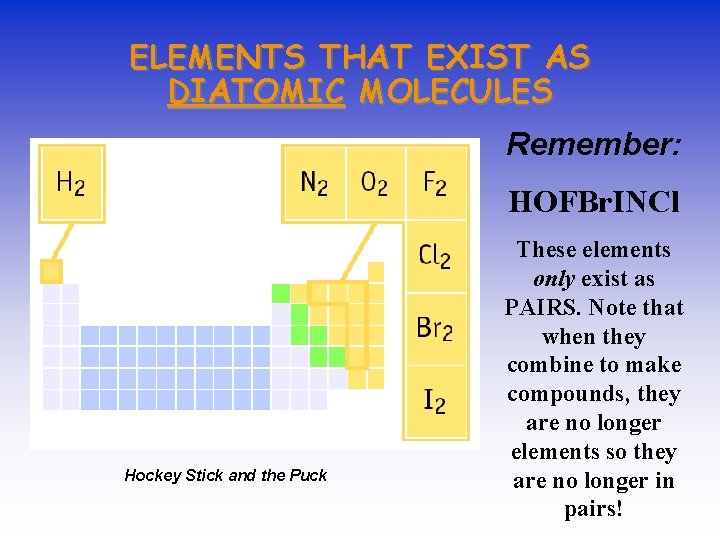
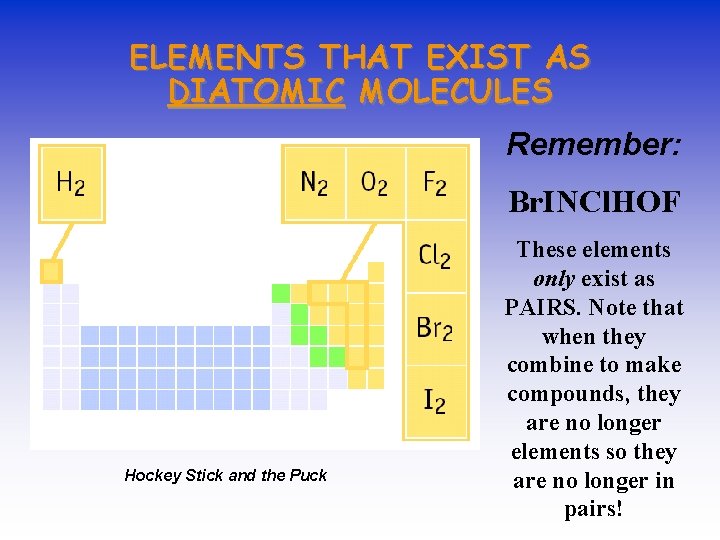
ELEMENTS THAT EXIST AS DIATOMIC MOLECULES Remember: HOFBr. INCl Hockey Stick and the Puck These elements only exist as PAIRS. Note that when they combine to make compounds, they are no longer elements so they are no longer in pairs!

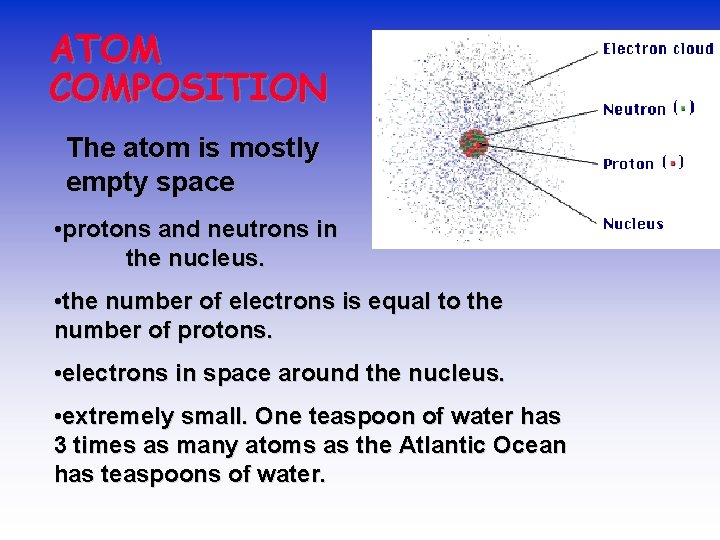
ATOM COMPOSITION The atom is mostly empty space • protons and neutrons in the nucleus. • the number of electrons is equal to the number of protons. • electrons in space around the nucleus. • extremely small. One teaspoon of water has 3 times as many atoms as the Atlantic Ocean has teaspoons of water.

ATOMIC COMPOSITION • Protons (p+) – – – + electrical charge mass = 1. 672623 x 10 -24 g relative mass = 1. 007 atomic mass units (amu) but we can round to 1 • Electrons (e-) negative electrical charge – relative mass = 0. 0005 amu but we can round to 0 – • Neutrons (no) no electrical charge – mass = 1. 009 amu but we can round to 1 –

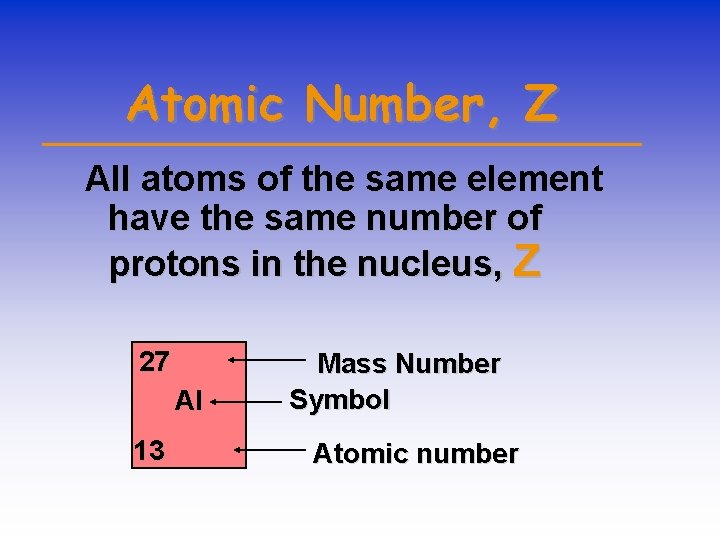
Atomic Number, Z All atoms of the same element have the same number of protons in the nucleus, Z 27 Al 13 Mass Number Symbol Atomic number

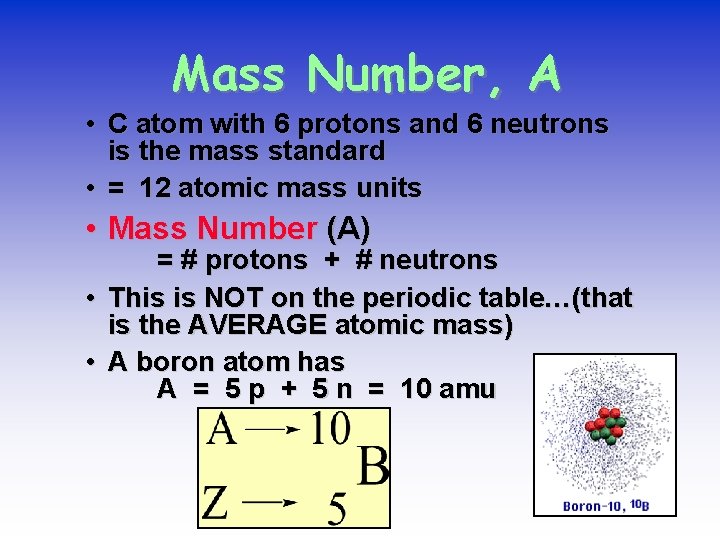
Mass Number, A • C atom with 6 protons and 6 neutrons is the mass standard • = 12 atomic mass units • Mass Number (A) • • = # protons + # neutrons This is NOT on the periodic table…(that is the AVERAGE atomic mass) A boron atom has A = 5 p + 5 n = 10 amu

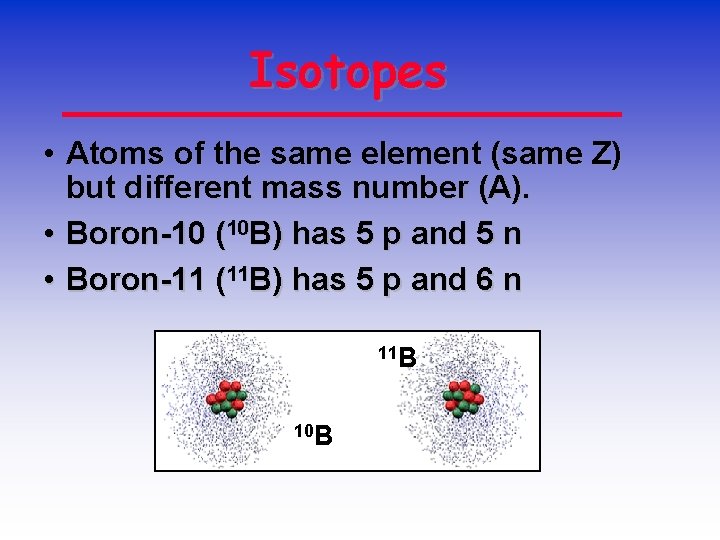
Isotopes • Atoms of the same element (same Z) but different mass number (A). • Boron-10 (10 B) has 5 p and 5 n • Boron-11 (11 B) has 5 p and 6 n 11 B 10 B

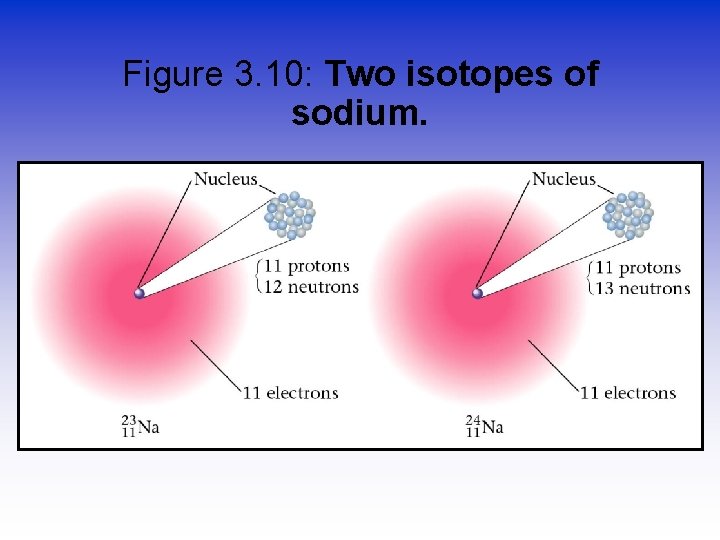
Figure 3. 10: Two isotopes of sodium.

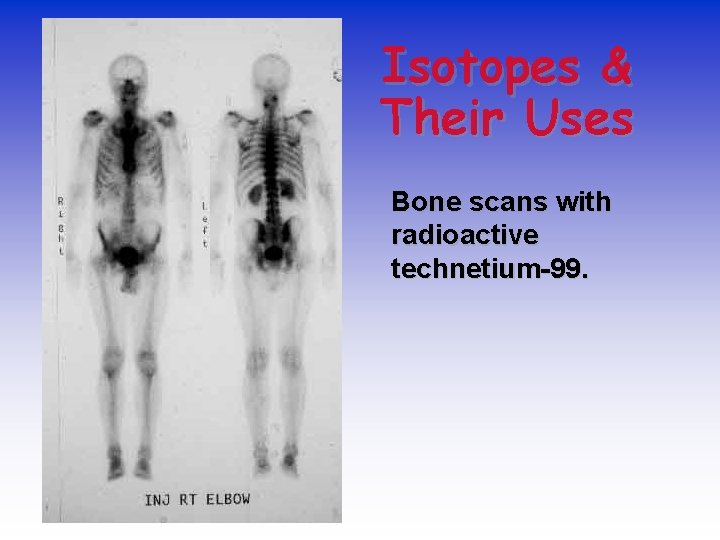
Isotopes & Their Uses Bone scans with radioactive technetium-99.

Isotopes & Their Uses The tritium content of ground water is used to discover the source of the water, for example, in municipal water or the source of the steam from a volcano.

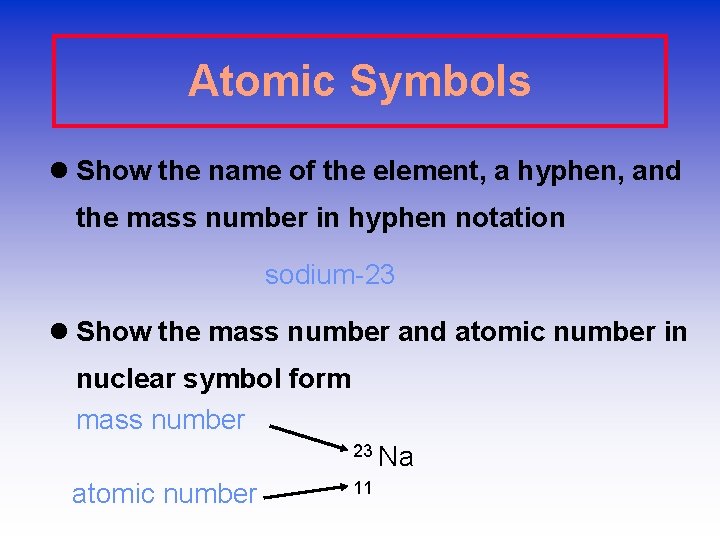
Atomic Symbols l Show the name of the element, a hyphen, and the mass number in hyphen notation sodium-23 l Show the mass number and atomic number in nuclear symbol form mass number 23 Na atomic number 11

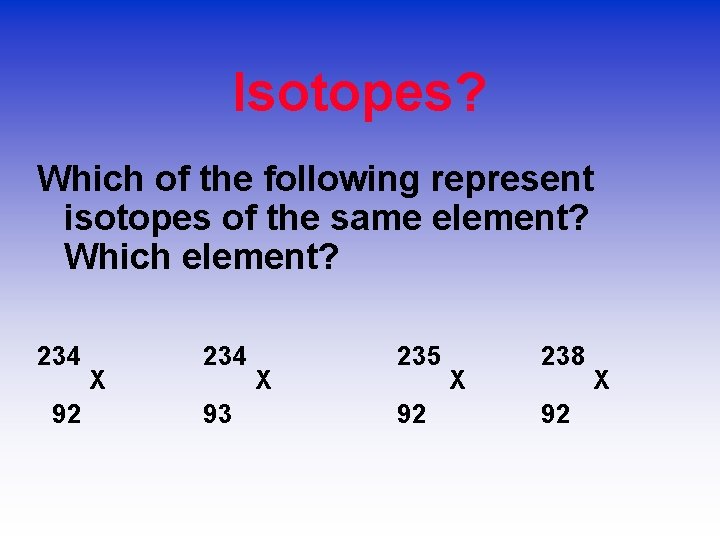
Isotopes? Which of the following represent isotopes of the same element? Which element? 234 92 X 234 X 93 235 92 X 238 92 X

Counting Protons, Neutrons, and Electrons • Protons: Atomic Number (from periodic table) • Neutrons: Mass Number minus the number of protons (mass number is protons and neutrons because the mass of electrons is negligible) • Electrons: – If it’s an atom, the protons and electrons must be the SAME so that it is has a net charge of zero (equal numbers of + and -) – If it does NOT have an equal number of electrons, it is not an atom, it is an ION. For each negative charge, add an extra electron. For each positive charge, subtract an electron (Don’t add a proton!!! That changes the element!)

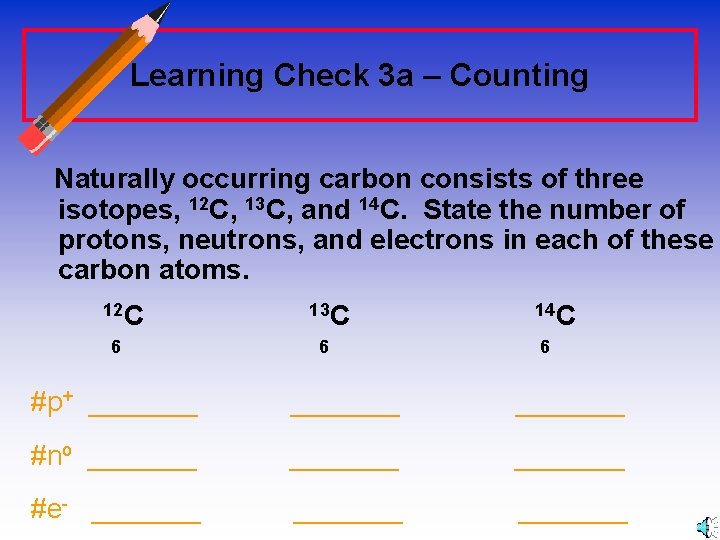
Learning Check 3 a – Counting Naturally occurring carbon consists of three isotopes, 12 C, 13 C, and 14 C. State the number of protons, neutrons, and electrons in each of these carbon atoms. 12 C 13 C 14 C 6 6 #p+ _______ #no _______ #e- _______

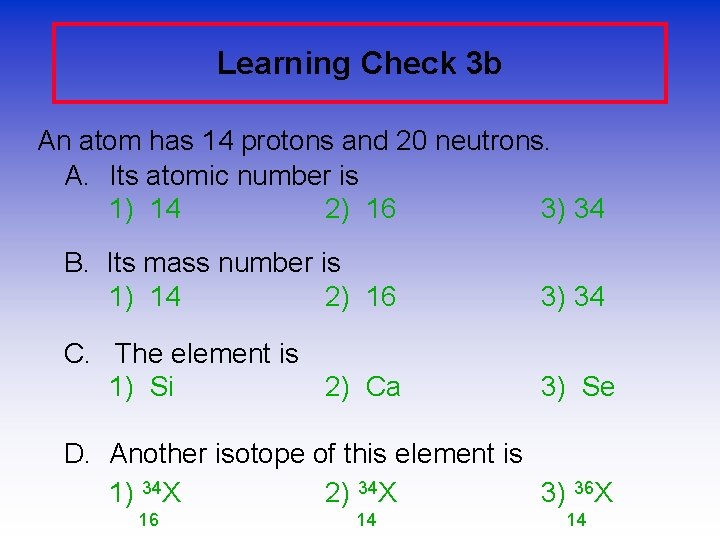
Learning Check 3 b An atom has 14 protons and 20 neutrons. A. Its atomic number is 1) 14 2) 16 3) 34 B. Its mass number is 1) 14 2) 16 3) 34 C. The element is 1) Si 2) Ca 3) Se D. Another isotope of this element is 1) 34 X 2) 34 X 3) 36 X 16 14 14

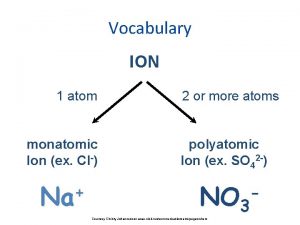
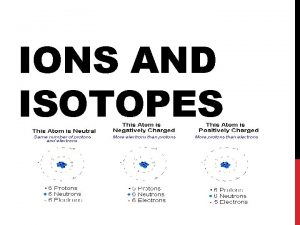
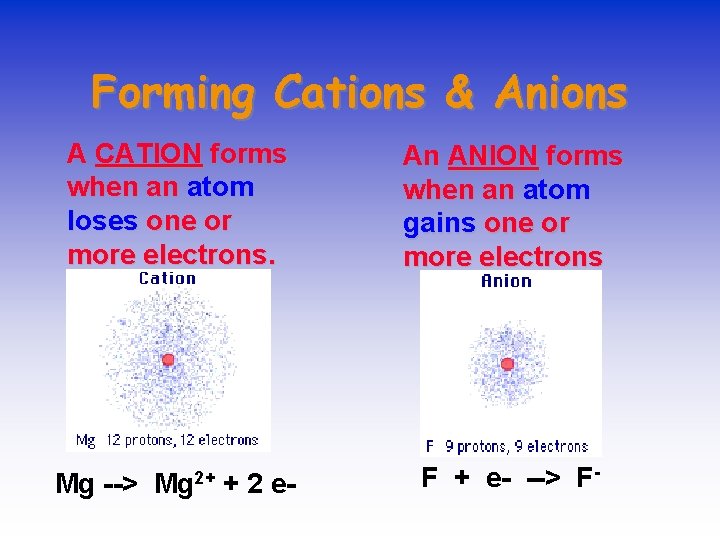
IONS • IONS are atoms or groups of atoms with a positive or negative charge. • Taking away an electron from an atom gives a CATION with a positive charge • Adding an electron to an atom gives an ANION with a negative charge. • To tell the difference between an atom and an ion, look to see if there is a charge in the superscript! Examples: Na+ Ca+2 I- O-2 Na Ca I O

Forming Cations & Anions A CATION forms when an atom loses one or more electrons. An ANION forms when an atom gains one or more electrons Mg --> Mg 2+ + 2 e- F + e- --> F-

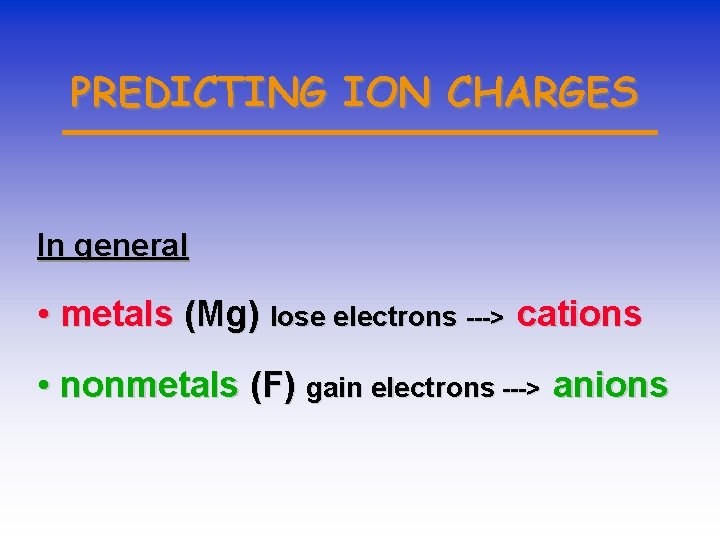
PREDICTING ION CHARGES In general • metals (Mg) lose electrons ---> cations • nonmetals (F) gain electrons ---> anions

Charges on Common Ions -4 -3 -2 -1 +1 +2 +3 By losing or gaining e-, atom has same number of e-’s as nearest Group 8 A atom.

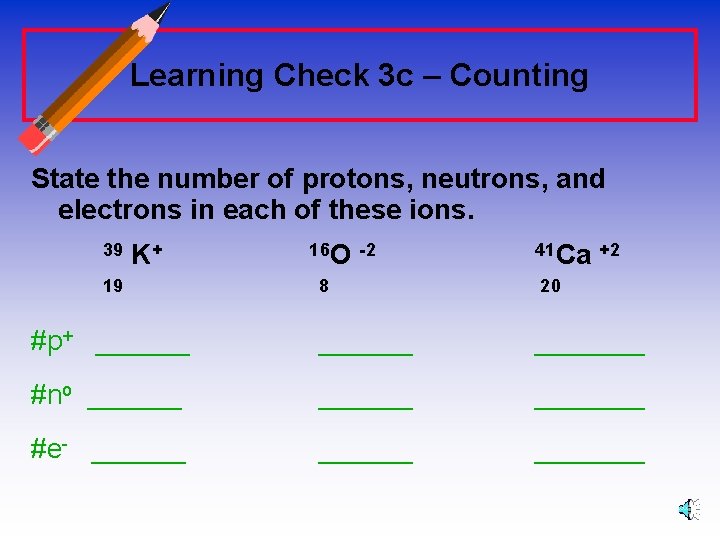
Learning Check 3 c – Counting State the number of protons, neutrons, and electrons in each of these ions. 39 K+ 19 16 O -2 8 41 Ca +2 20 #p+ _______ #no _______ #e- _______

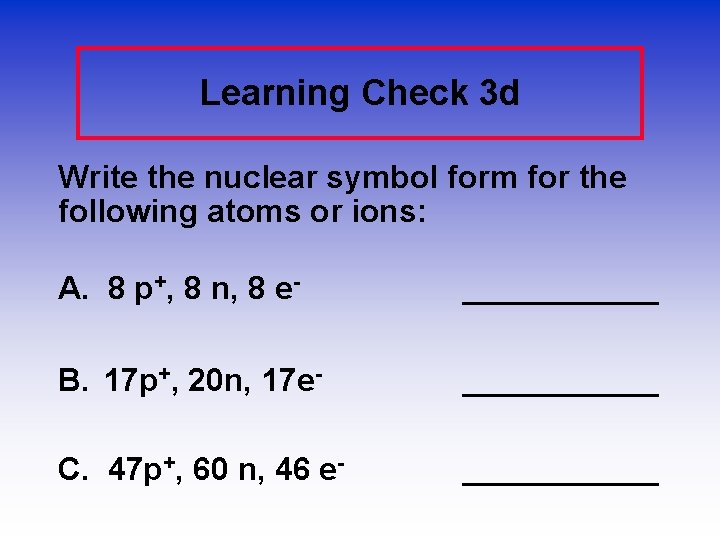
Learning Check 3 d Write the nuclear symbol form for the following atoms or ions: A. 8 p+, 8 n, 8 e- ______ B. 17 p+, 20 n, 17 e- ______ C. 47 p+, 60 n, 46 e- ______

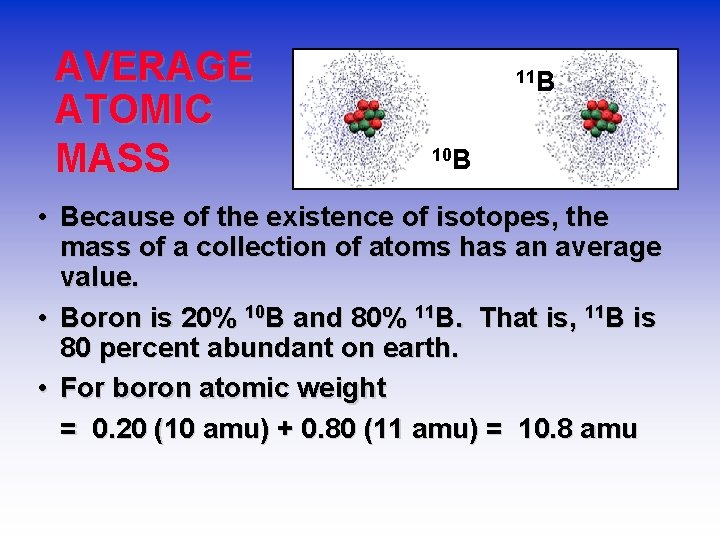
AVERAGE ATOMIC MASS 11 B 10 B • Because of the existence of isotopes, the mass of a collection of atoms has an average value. • Boron is 20% 10 B and 80% 11 B. That is, 11 B is 80 percent abundant on earth. • For boron atomic weight = 0. 20 (10 amu) + 0. 80 (11 amu) = 10. 8 amu

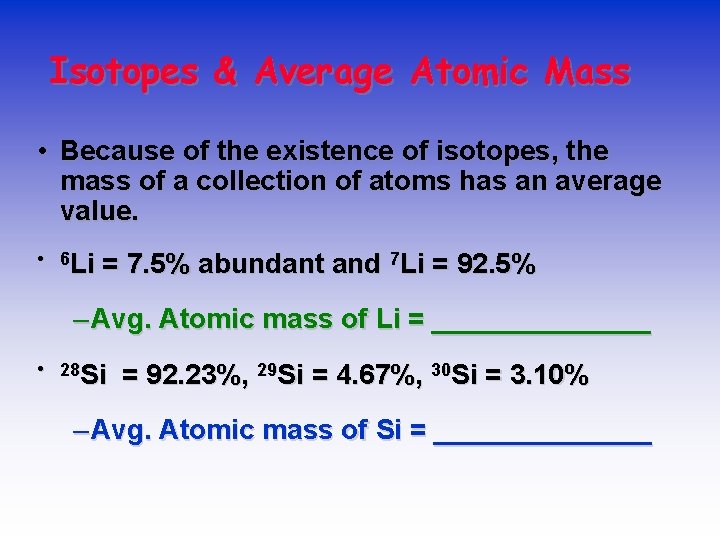
Isotopes & Average Atomic Mass • Because of the existence of isotopes, the mass of a collection of atoms has an average value. • 6 Li = 7. 5% abundant and 7 Li = 92. 5% – Avg. Atomic mass of Li = _______ • 28 Si = 92. 23%, 29 Si = 4. 67%, 30 Si = 3. 10% – Avg. Atomic mass of Si = _______

Lesson 4 Organizing the Periodic Table Essential Question How are elements grouped in the Periodic Table? Objectives • To identify similar properties of elements in groups and periods.

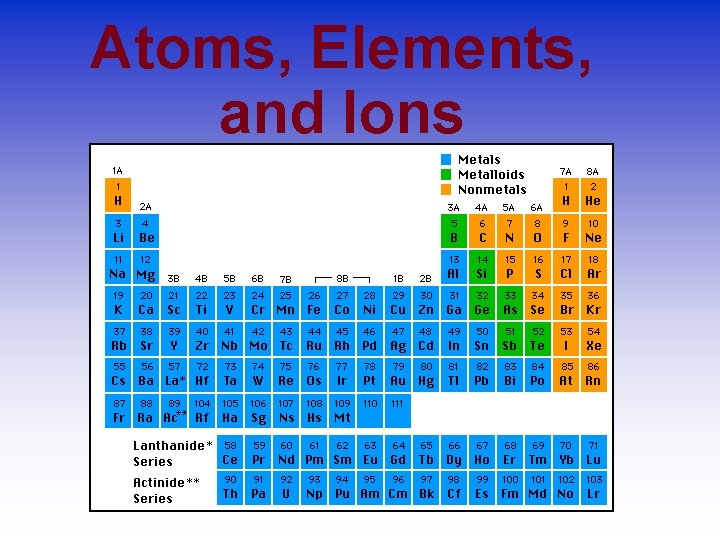
Elements Element- atoms having an identical number of protons in each nucleus. Elements cannot be reduced to simpler substances by normal chemical means. • Elements are organized into the Periodic Table of Elements by their Atomic Number • 1 H 2 He 3 Li 4 Be 5 B 6 C 7 N

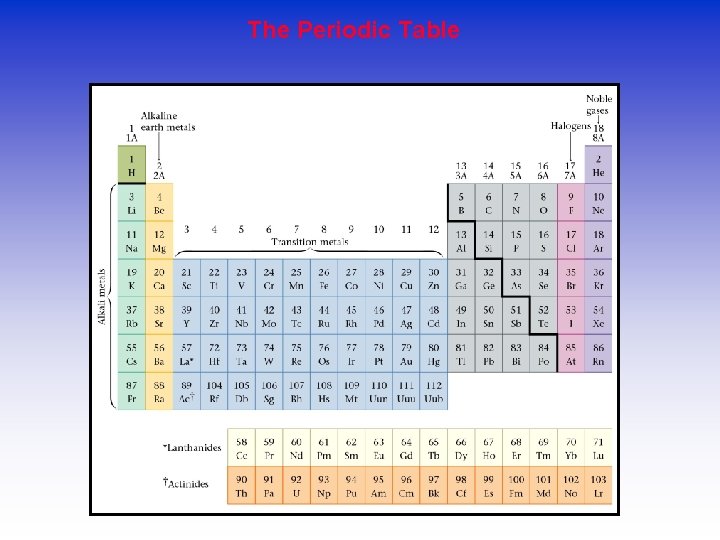
The Periodic Table

Periods in the Periodic Table

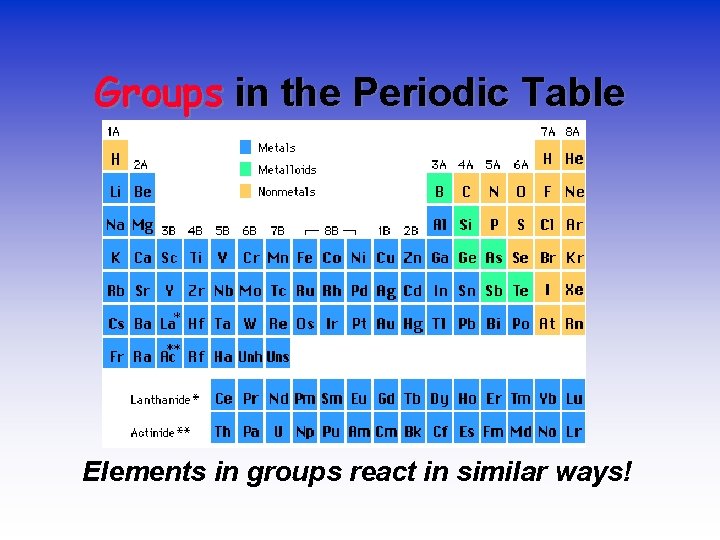
Groups in the Periodic Table Elements in groups react in similar ways!

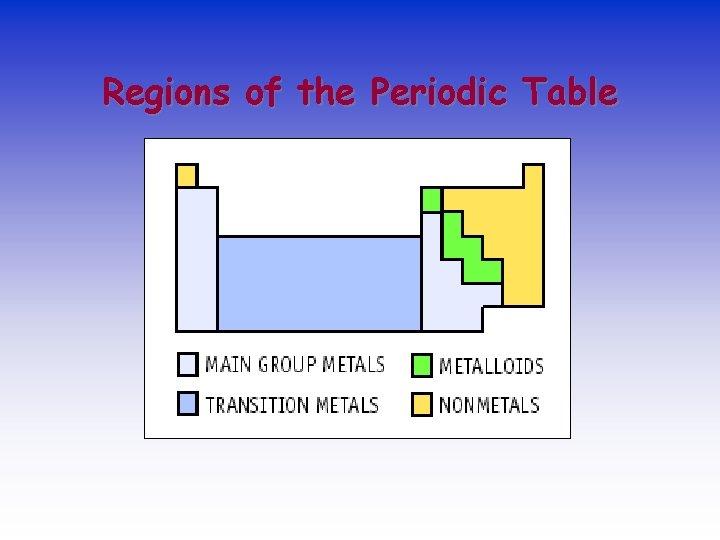
Regions of the Periodic Table

Group 1 A: Alkali Metals Reaction of potassium + H 2 O Cutting sodium metal

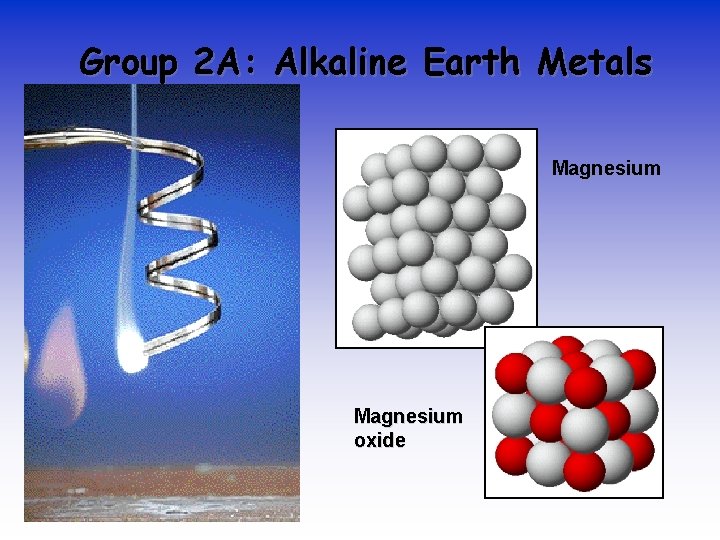
Group 2 A: Alkaline Earth Metals Magnesium oxide

Group 7 A: The Halogens (salt makers) F, Cl, Br, I, At

Group 8 A: The Noble (Inert) Gases He, Ne, Ar, Kr, Xe, Rn • Lighter than air balloons • “Neon” signs • Very Unreactive because they have full electron levels Xe. OF 4

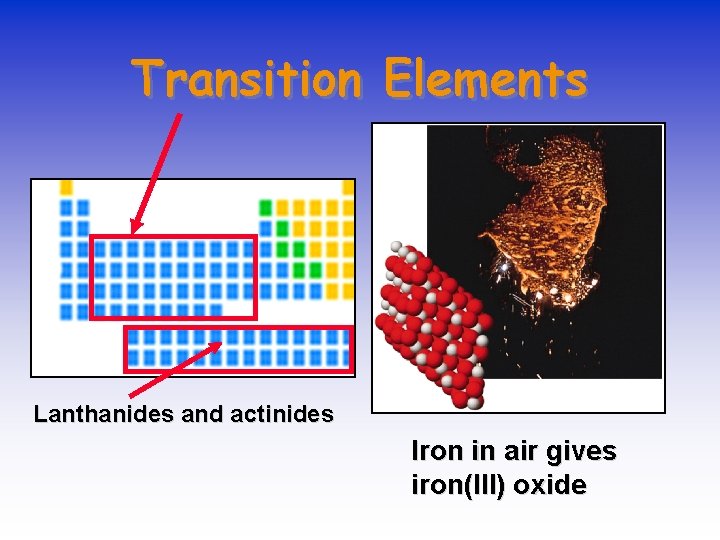
Transition Elements Lanthanides and actinides Iron in air gives iron(III) oxide

ELEMENTS THAT EXIST AS DIATOMIC MOLECULES Remember: Br. INCl. HOF Hockey Stick and the Puck These elements only exist as PAIRS. Note that when they combine to make compounds, they are no longer elements so they are no longer in pairs!

Compounds • Compounds are… – Substances that are made from atoms of two or more different elements chemically bonded together. – Ex: Na. Cl, Ca. CO 3

Molecules • Molecules are… – Substances that are made from atoms of two or more alike or different element chemically bonded together. – Ex: O 2, H 2 (diatomics), Na. Cl
 Carbon trichloride
Carbon trichloride Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules States that atoms ions and molecules must collide to react
States that atoms ions and molecules must collide to react Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Positive ions are atoms that have
Positive ions are atoms that have Atoms or ions are considered isoelectronic if
Atoms or ions are considered isoelectronic if Regents periodic table
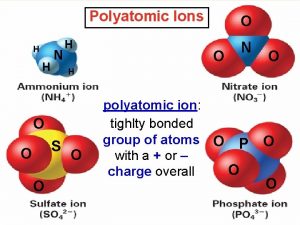
Regents periodic table Definition polyatomic ion
Definition polyatomic ion Spectator ions in chemistry
Spectator ions in chemistry Ap chemistry electronic structure of atoms
Ap chemistry electronic structure of atoms Study jams mixtures
Study jams mixtures Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 chemistry in biology
Chapter 6 chemistry in biology Chapter 6 section 1 atoms elements and compounds
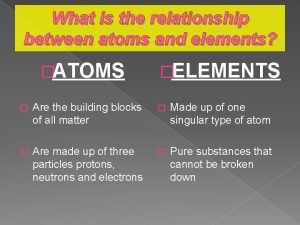
Chapter 6 section 1 atoms elements and compounds What is the relationship between atoms and elements
What is the relationship between atoms and elements Element vs molecule vs atom
Element vs molecule vs atom Www.chem.purdue/gchelp/atoms/elements.html
Www.chem.purdue/gchelp/atoms/elements.html Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa yêu trần thế
Chúa yêu trần thế Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc cc
V cc cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Các số nguyên tố
Các số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Monatomic element example
Monatomic element example Polytomic ion
Polytomic ion Charges of compounds
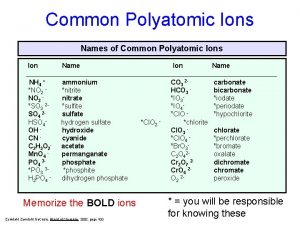
Charges of compounds 89. common polyatomic ions
89. common polyatomic ions Positive negative attract each other
Positive negative attract each other Srim the stopping and range of ions in matter
Srim the stopping and range of ions in matter Multivalent ionic compounds worksheet
Multivalent ionic compounds worksheet Freezing point chapter 13
Freezing point chapter 13 Chemistry formulas list
Chemistry formulas list Ions and ionic bonding cornell doodle notes
Ions and ionic bonding cornell doodle notes Elements of environmental chemistry
Elements of environmental chemistry Ap chemistry chapter 7 periodic properties of the elements
Ap chemistry chapter 7 periodic properties of the elements Overhead allocation
Overhead allocation Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Counting atoms examples
Counting atoms examples Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Properties of atoms and the periodic table
Properties of atoms and the periodic table The lowest allowable energy state of an atom
The lowest allowable energy state of an atom Mit center for bits and atoms
Mit center for bits and atoms Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Balancing equations counting atoms
Balancing equations counting atoms 3bacl2 counting atoms
3bacl2 counting atoms Atoms and radioactivity
Atoms and radioactivity Counting atoms and balancing equations worksheet
Counting atoms and balancing equations worksheet Isotopes pogil
Isotopes pogil Quantum theory and the electronic structure of atoms
Quantum theory and the electronic structure of atoms Formula in chemistry
Formula in chemistry Chemistry language
Chemistry language Urea ion
Urea ion Sulfate anion test
Sulfate anion test Writing formulas criss cross method examples
Writing formulas criss cross method examples Qulitative analysis
Qulitative analysis What is a spectator ion
What is a spectator ion Polyhalide examples
Polyhalide examples