The Dow Chemical Company About the Company F

The Dow Chemical Company

About the Company F Founded in 1897 by Herbert H. Dow in Midland, Michigan F Fifth largest chemical company in the world, with sales of more than $20 billion F 94 manufacturing sites in 30 countries F More than 39, 500 employees around the world

Liquid Separations FA global business within Specialty Chemicals F Dowex* Ion Exchange Resins celebrated 60 years experience. They are the conerstone. F Film. Tec* Membranes are our fastest growing product line. Our 30 th year in membranes
THE DOW LIQUID SEPARATIONS Manufacturing Sites Minneapolis Stade(Germany) Midland Fombio(Italy) Freeport Pacific - US/EUR sourcing
THE DOW LIQUID SEPARATIONS R&D, TS&D Locations Minneapolis Midland Rheinmunster Gotemba Pacific - Japan/Technical center

THE LIQUID SEPARATIONS Products 1 - IER F Resin Materials – Ion Exchange Resins (R) F Dowex Gaussian Resins (HCR-S, SAR, SBR-P) (R) F Dowex UPS (Uniform) Resins – Marathon(R) Monospere(R) – Adsorbent Resins (R) F Dowex Optipore Resins F Technology – UPCORE License (R) Counter Current
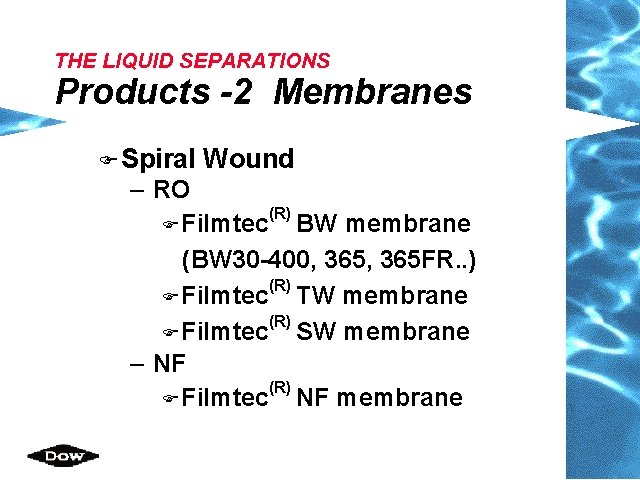
THE LIQUID SEPARATIONS Products -2 Membranes F Spiral Wound – RO (R) F Filmtec BW membrane (BW 30 -400, 365 FR. . ) (R) F Filmtec TW membrane (R) F Filmtec SW membrane – NF (R) F Filmtec NF membrane
THE DOW LIQUID SEPARATIONS Manufacturing Sites Minneapolis Stade(Germany) Midland Fombio(Italy) Freeport Pacific - US/EUR sourcing
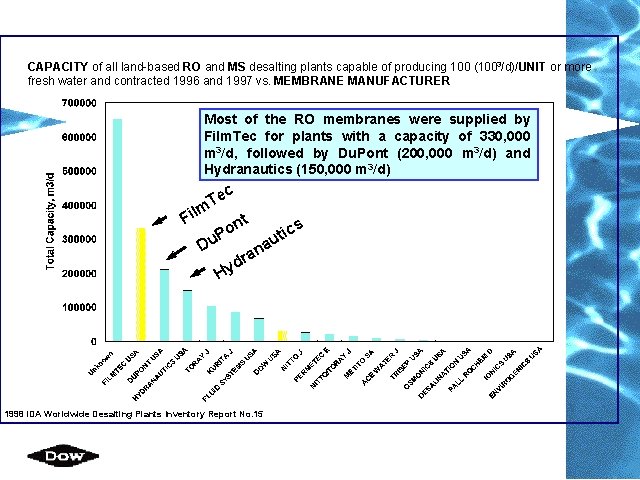
CAPACITY of all land-based RO and MS desalting plants capable of producing 100 (1003/d)/UNIT or more fresh water and contracted 1996 and 1997 vs. MEMBRANE MANUFACTURER Most of the RO membranes were supplied by Film. Tec for plants with a capacity of 330, 000 m 3/d, followed by Du. Pont (200, 000 m 3/d) and Hydranautics (150, 000 m 3/d) c Te m il F nt o cs i t P au Du n a dr y H 1998 IDA Worldwide Desalting Plants Inventory Report No. 15
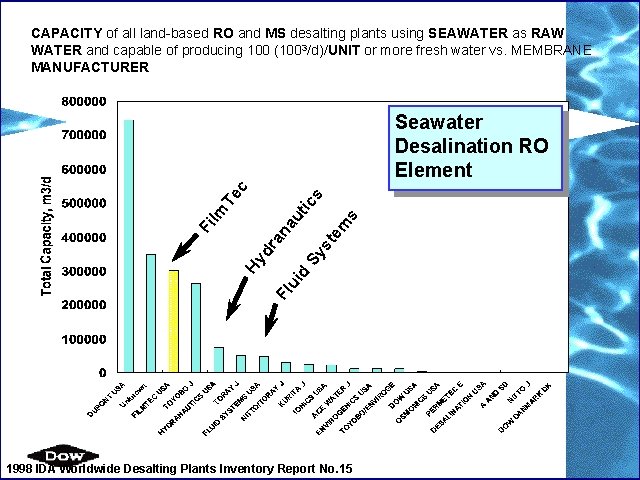
CAPACITY of all land-based RO and MS desalting plants using SEAWATER as RAW WATER and capable of producing 100 (1003/d)/UNIT or more fresh water vs. MEMBRANE MANUFACTURER s em Sy st d Fl ui Hy dr a na u Fi lm tic s Te c Seawater Desalination RO Element 1998 IDA Worldwide Desalting Plants Inventory Report No. 15
Fundamentals of Membranes and Reverse Osmosis
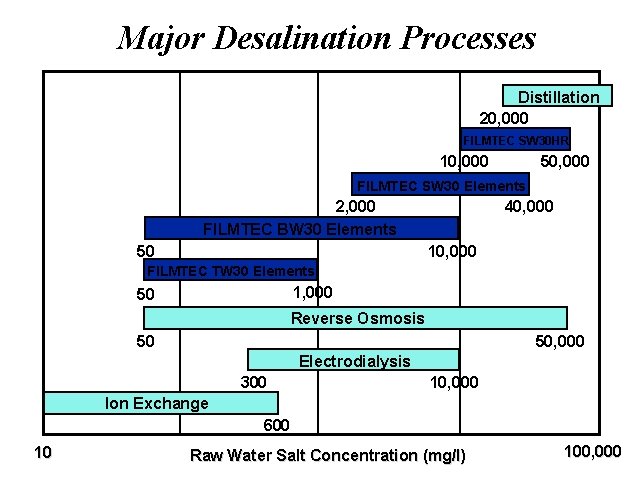
Major Desalination Processes Distillation 20, 000 FILMTEC SW 30 HR 10, 000 50, 000 FILMTEC SW 30 Elements 2, 000 FILMTEC BW 30 Elements 50 40, 000 10, 000 FILMTEC TW 30 Elements 1, 000 50 Reverse Osmosis 50 50, 000 Electrodialysis 300 10, 000 Ion Exchange 600 10 Raw Water Salt Concentration (mg/l) 100, 000
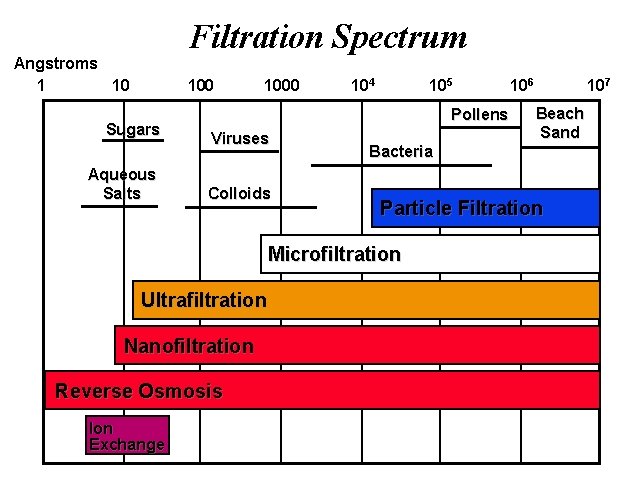
Filtration Spectrum Angstroms 1 10 100 Sugars Aqueous Salts 1000 104 105 Pollens Viruses Colloids Nanofiltration Reverse Osmosis Ion Exchange 107 Beach Sand Bacteria Particle Filtration Microfiltration Ultrafiltration 106

Microfiltration Involves F Separation mechanism based on pore size (» 0. 1 to 1 micron) F Pressure requirements independent of osmotic pressure of solution F Usually “full-flow” rather than crossflow
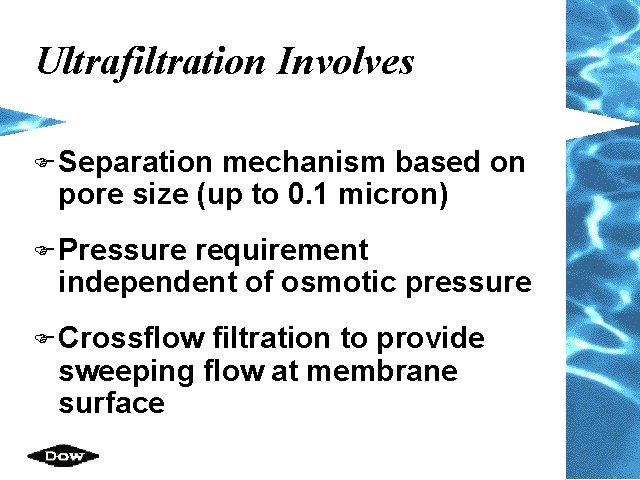
Ultrafiltration Involves F Separation mechanism based on pore size (up to 0. 1 micron) F Pressure requirement independent of osmotic pressure F Crossflow filtration to provide sweeping flow at membrane surface
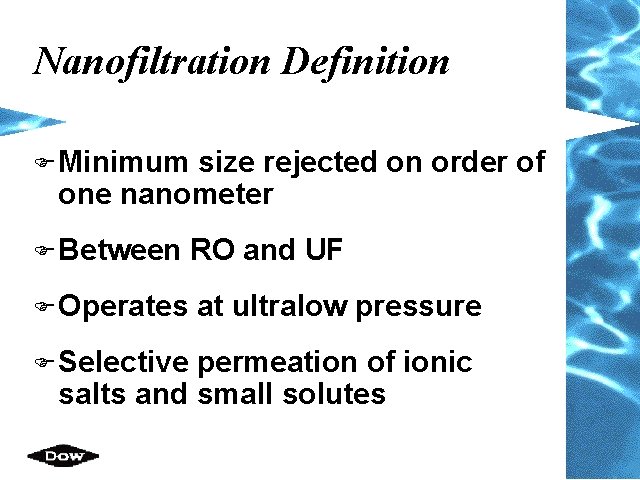
Nanofiltration Definition F Minimum size rejected on order of one nanometer F Between RO and UF F Operates at ultralow pressure F Selective permeation of ionic salts and small solutes
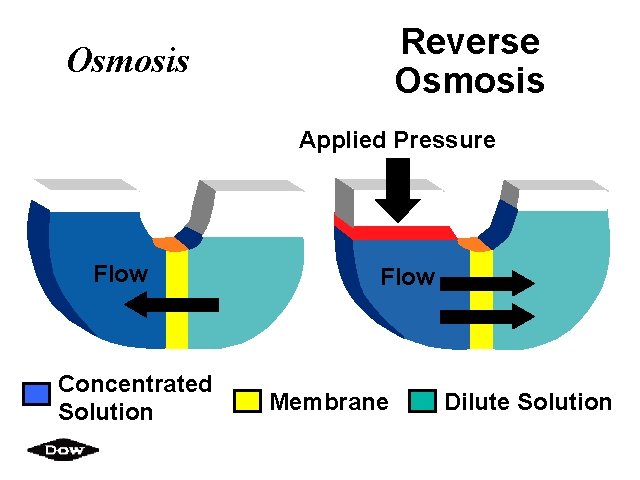
Reverse Osmosis Applied Pressure Flow Concentrated Solution Flow Membrane Dilute Solution
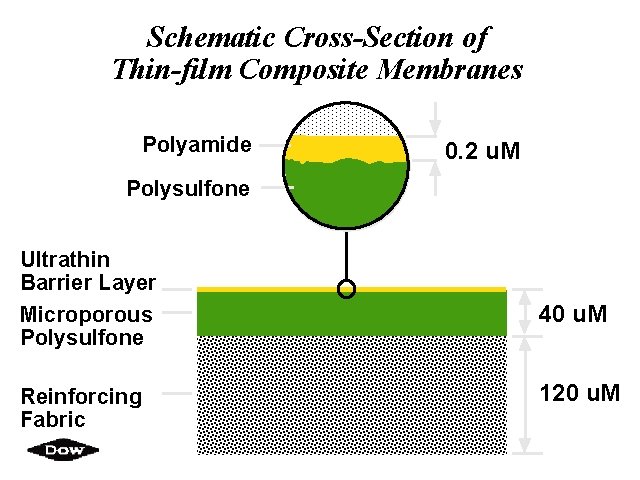
Schematic Cross-Section of Thin-film Composite Membranes Polyamide 0. 2 u. M Polysulfone Ultrathin Barrier Layer Microporous Polysulfone Reinforcing Fabric 40 u. M 120 u. M
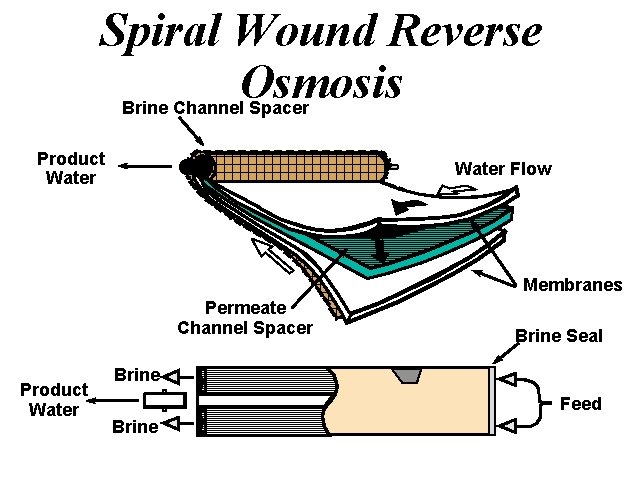
Spiral Wound Reverse Osmosis Brine Channel Spacer Product Water Flow Membranes Permeate Channel Spacer Product Water Brine Seal Brine Feed Brine
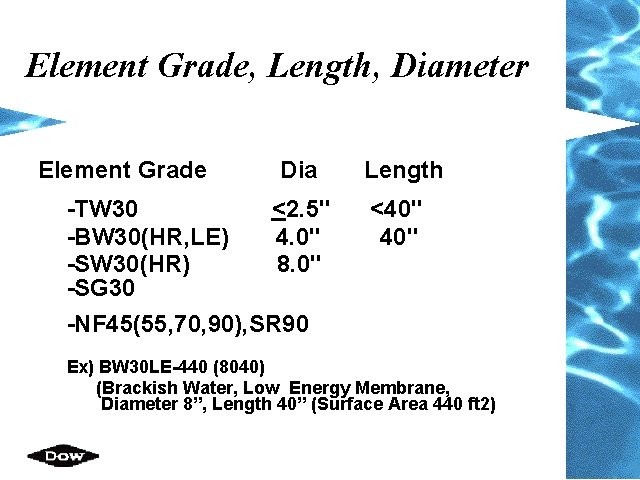
Element Grade, Length, Diameter Element Grade Dia -TW 30 <2. 5" -BW 30(HR, LE) 4. 0" -SW 30(HR) 8. 0" -SG 30 -NF 45(55, 70, 90), SR 90 Length <40" Ex) BW 30 LE-440 (8040) (Brackish Water, Low Energy Membrane, Diameter 8”, Length 40” (Surface Area 440 ft 2)
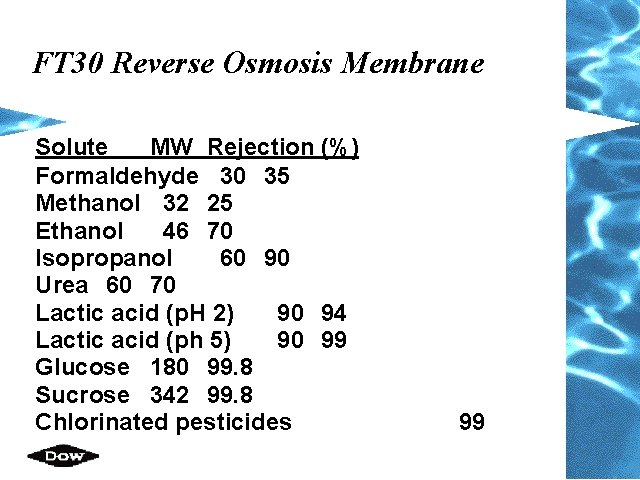
FT 30 Reverse Osmosis Membrane Solute MW Rejection (%) Formaldehyde 30 35 Methanol 32 25 Ethanol 46 70 Isopropanol 60 90 Urea 60 70 Lactic acid (p. H 2) 90 94 Lactic acid (ph 5) 90 99 Glucose 180 99. 8 Sucrose 342 99. 8 Chlorinated pesticides 99
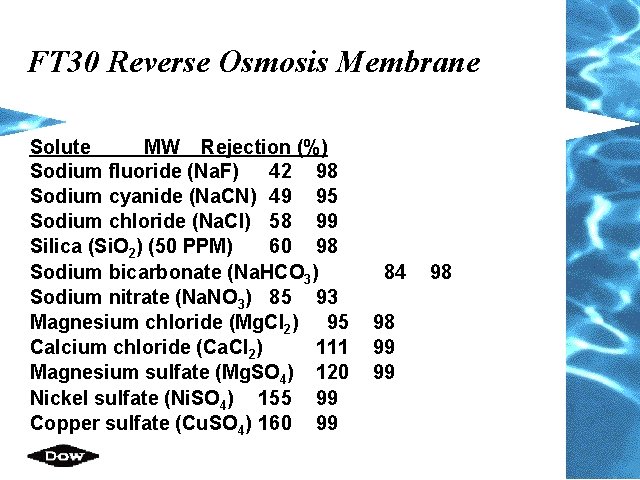
FT 30 Reverse Osmosis Membrane Solute MW Rejection (%) Sodium fluoride (Na. F) 42 98 Sodium cyanide (Na. CN) 49 95 Sodium chloride (Na. Cl) 58 99 Silica (Si. O 2) (50 PPM) 60 98 Sodium bicarbonate (Na. HCO 3) Sodium nitrate (Na. NO 3) 85 93 Magnesium chloride (Mg. Cl 2) 95 Calcium chloride (Ca. Cl 2) 111 Magnesium sulfate (Mg. SO 4) 120 Nickel sulfate (Ni. SO 4) 155 99 Copper sulfate (Cu. SO 4) 160 99 84 98 99 99 98
THE DOW LIQUID SEPARATIONS Manufacturing Sites Minneapolis Stade(Germany) Midland Fombio(Italy) Freeport Pacific - US/EUR sourcing
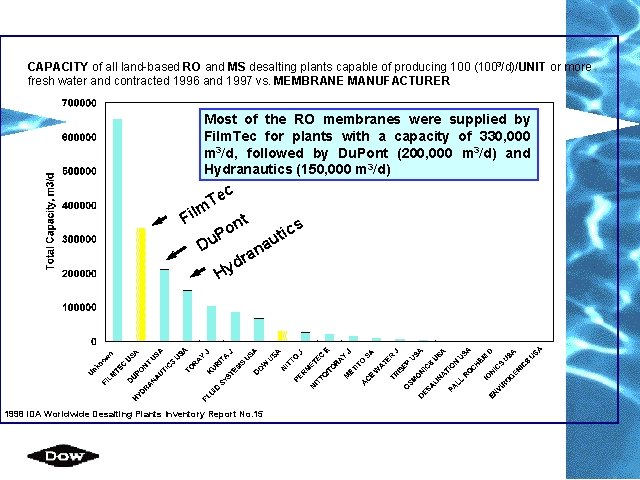
CAPACITY of all land-based RO and MS desalting plants capable of producing 100 (1003/d)/UNIT or more fresh water and contracted 1996 and 1997 vs. MEMBRANE MANUFACTURER Most of the RO membranes were supplied by Film. Tec for plants with a capacity of 330, 000 m 3/d, followed by Du. Pont (200, 000 m 3/d) and Hydranautics (150, 000 m 3/d) c Te m il F nt o cs i t P au Du n a dr y H 1998 IDA Worldwide Desalting Plants Inventory Report No. 15

FILMTEC RO FILMTEC* Membranes
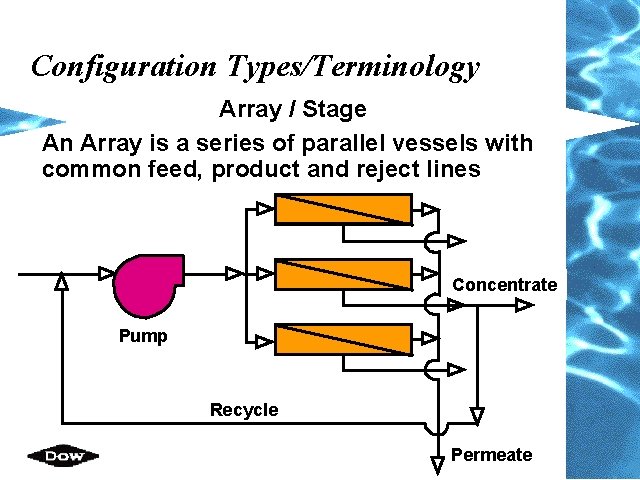
Configuration Types/Terminology Array / Stage An Array is a series of parallel vessels with common feed, product and reject lines Concentrate Pump Recycle Permeate
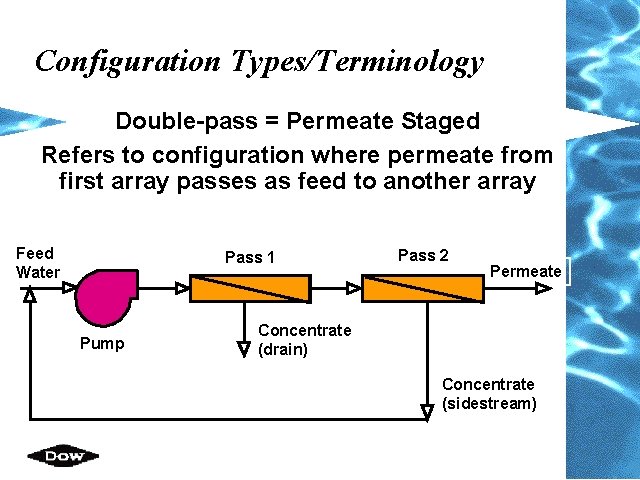
Configuration Types/Terminology Double-pass = Permeate Staged Refers to configuration where permeate from first array passes as feed to another array Feed Water Pass 1 Pump Pass 2 Permeate Concentrate (drain) Concentrate (sidestream)
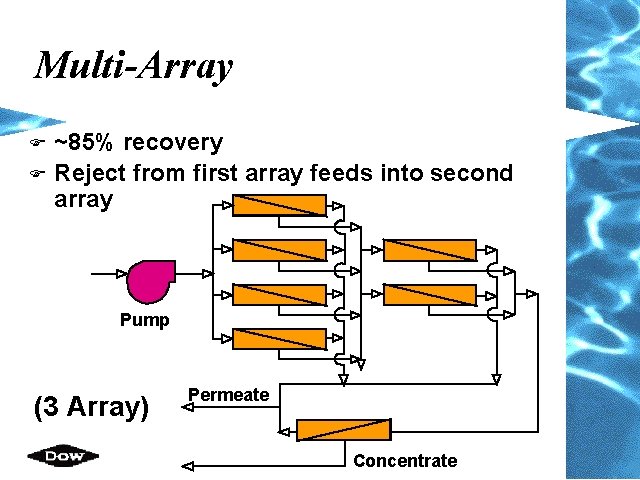
Multi-Array F F ~85% recovery Reject from first array feeds into second array Pump (3 Array) Permeate Concentrate
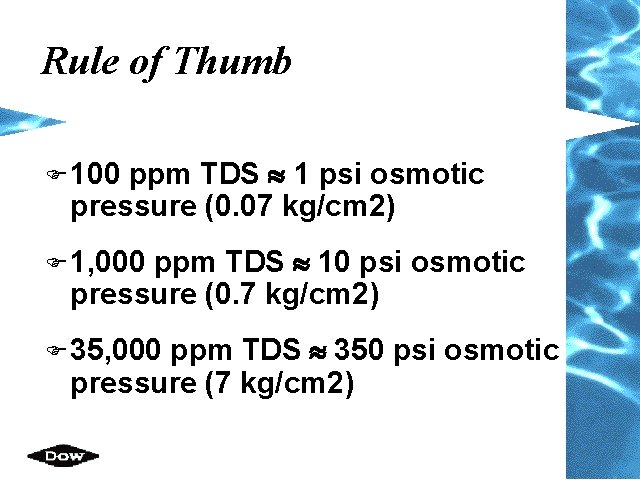
Rule of Thumb ppm TDS » 1 psi osmotic pressure (0. 07 kg/cm 2) F 100 ppm TDS » 10 psi osmotic pressure (0. 7 kg/cm 2) F 1, 000 ppm TDS » 350 psi osmotic pressure (7 kg/cm 2) F 35, 000
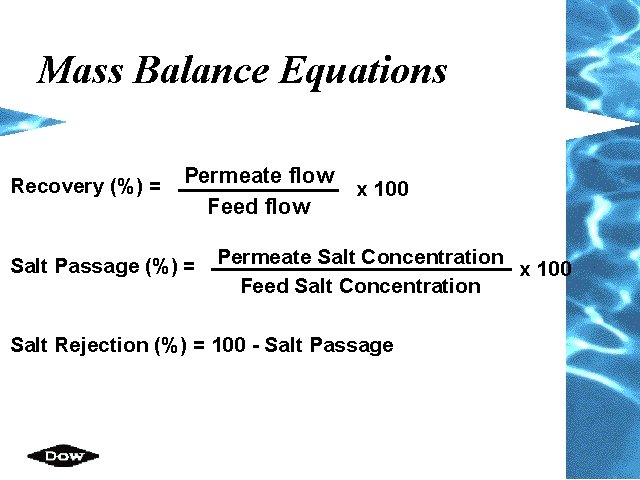
Mass Balance Equations Recovery (%) = Permeate flow Feed flow Salt Passage (%) = x 100 Permeate Salt Concentration x 100 Feed Salt Concentration Salt Rejection (%) = 100 - Salt Passage
Factors Which Affect Performance of Membranes F Feedwater pressure F Feedwater temperature F Feedwater concentration F Increased recovery
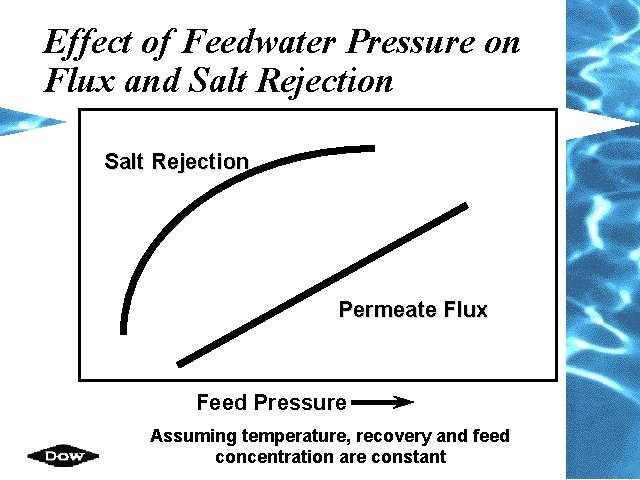
Effect of Feedwater Pressure on Flux and Salt Rejection Permeate Flux Feed Pressure Assuming temperature, recovery and feed concentration are constant
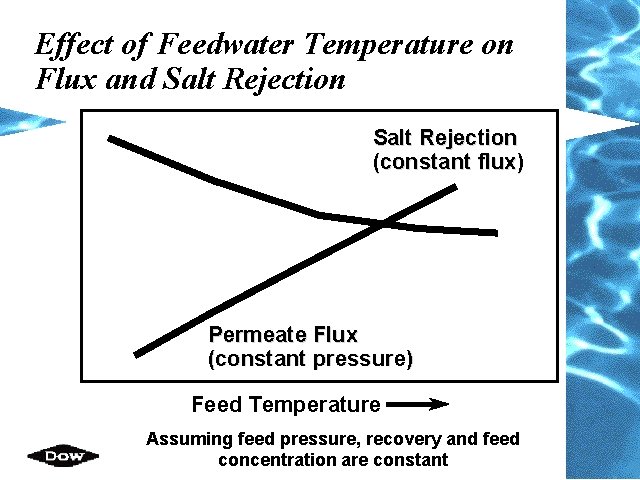
Effect of Feedwater Temperature on Flux and Salt Rejection (constant flux) Permeate Flux (constant pressure) Feed Temperature Assuming feed pressure, recovery and feed concentration are constant
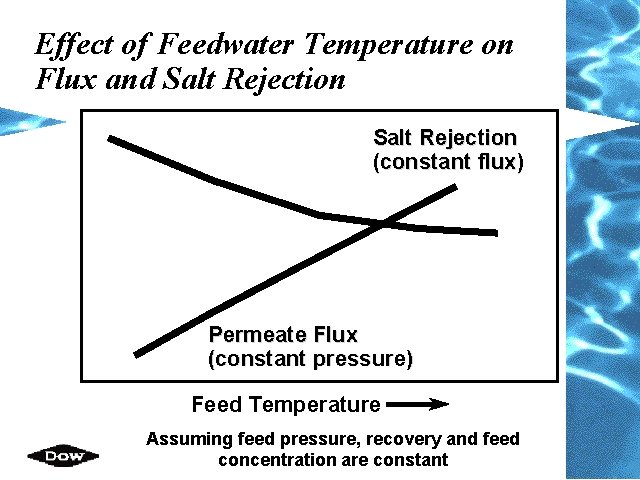
Effect of Feedwater Temperature on Flux and Salt Rejection (constant flux) Permeate Flux (constant pressure) Feed Temperature Assuming feed pressure, recovery and feed concentration are constant
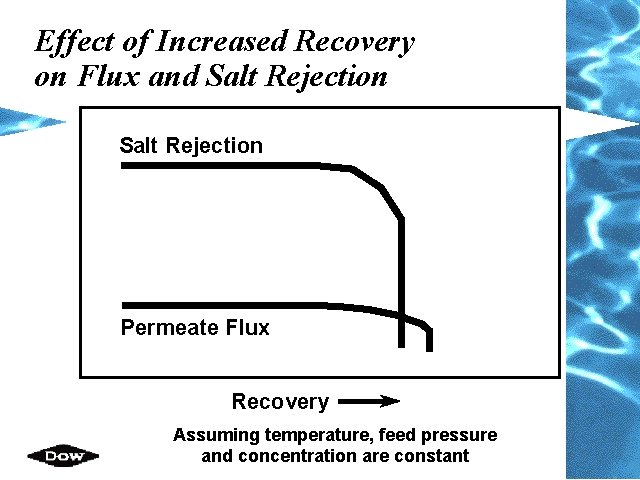
Effect of Increased Recovery on Flux and Salt Rejection Permeate Flux Recovery Assuming temperature, feed pressure and concentration are constant
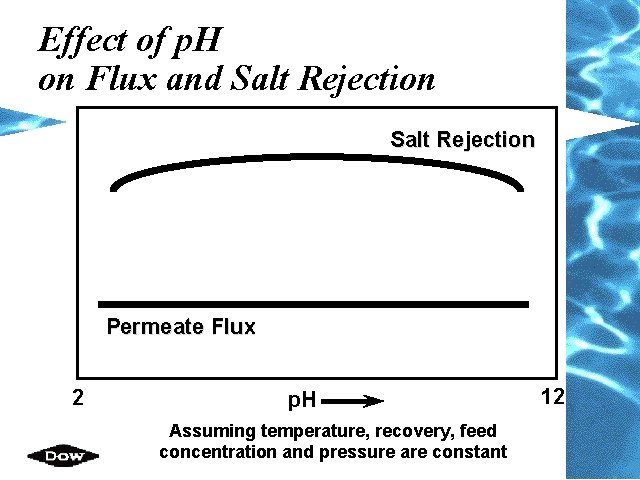
Effect of p. H on Flux and Salt Rejection Permeate Flux 2 p. H Assuming temperature, recovery, feed concentration and pressure are constant 12

Advanced RO Design Training 2/25/2021

EVALUATING FEEDWATER SOURCES F Groundwater F Surface water F Waste water F Seawater 2/25/2021
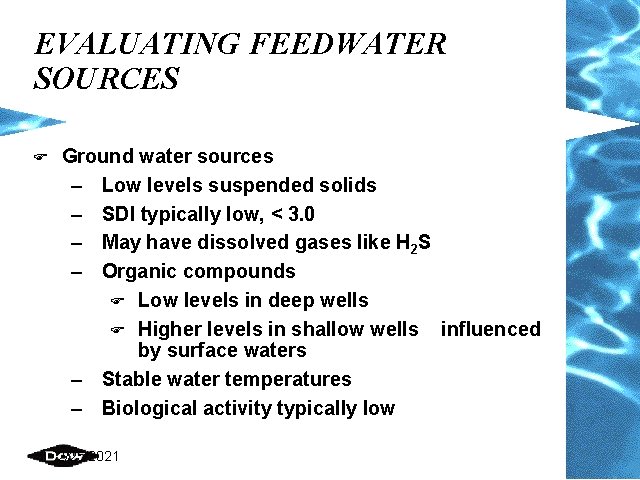
EVALUATING FEEDWATER SOURCES F Ground water sources – Low levels suspended solids – SDI typically low, < 3. 0 – May have dissolved gases like H 2 S – Organic compounds F Low levels in deep wells F Higher levels in shallow wells influenced by surface waters – Stable water temperatures – Biological activity typically low 2/25/2021
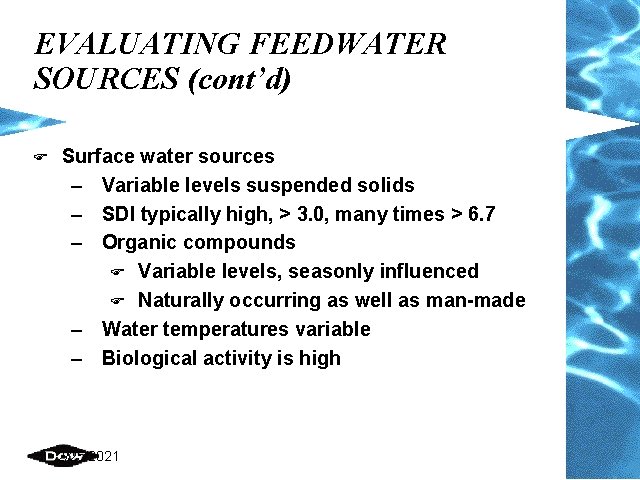
EVALUATING FEEDWATER SOURCES (cont’d) F Surface water sources – Variable levels suspended solids – SDI typically high, > 3. 0, many times > 6. 7 – Organic compounds F Variable levels, seasonly influenced F Naturally occurring as well as man-made – Water temperatures variable – Biological activity is high 2/25/2021
EVALUATING FEEDWATER SOURCES (cont’d) F Waste water sources – Variable levels suspended solids – SDI typically high, > 3. 0, many times > 6. 7 – Organic compounds F Variable levels, seasonly influenced F Naturally occurring as well as man-made – Water temperatures variable – Biological activity is very high 2/25/2021
EVALUATING FEEDWATER SOURCES (cont’d) F Seawater sources – Surface intake or beach wells – SDI depends on source F Suface intake > 3. 0 F Beach wells < 3. 0 – Organic compounds - typically low – Water temperatures variable (surface intake) – Biological activity is high (surface intake) 2/25/2021

EVALUATING FEEDWATER SOURCES F Feedwater information helps determine pretreatment unit operations Allows Dow to suggest the right or best element for the application Allows Dow to suggest the best design for low cost plus reliable operation 2/25/2021
PRETREATMENT CONSIDERATIONS F Filtration – Media filtration – Microfiltration – Ultrafiltration Dechlorination Scale Control 2/25/2021

PRETREATMENT CONSIDERATIONS F Media filtration – Reduction in colloidal, suspended and particulate material – SDI utilized to measure filtration effectiveness – Filters can be single or multi- media – Coagulants and flocculants sometimes dosed before filters to enhance filtration 2/25/2021

PRETREATMENT CONSIDERATIONS F Microfiltration (MF) – Produces better feedwater quality than media filtration – Higher capital cost – May allow for more aggressive RO membrane design – Expands the choices for RO membranes on difficult-to-treat water applications 2/25/2021

PRETREATMENT CONSIDERATIONS F Ultrafiltration (UF) – Produces better water quality than media filtration or MF – Higher capital cost – May allow for more aggressive RO membrane design – Expands the choices for RO membranes on difficult-to-treat water applications 2/25/2021
PRETREATMENT CONSIDERATIONS F Dechlorination – Granular Activated Carbon (GAC) F Removes chlorine very well F Source of bacterial contamination F Carbon fines – Sodium Meta-bisulfite (SMBS) F Reacts quickly with chlorine F Better choice in warm climates F Sulfate reducing bacteria 2/25/2021

PRETREATMENT CONSIDERATIONS F Scale control – Antiscalants F Designed for sulfate and silica scales F Can be formulated for water sources containing iron or aluminum F Dow does not approve products – Mineral acids F Typically HCl or H 2 SO 4 F Sometimes used with antiscalants – Ion exchange softening 2/25/2021
Other Factors to be Aware of … F Fouling factor concept – BW – 1. 0 initial to. 85 in 3 years – SW – 1. 0 initial to. 8 in 3 years
Other Factors to be Aware of … Feed Composition on System Recovery F Seawater recovery limitations – High osmotic pressure – Osmotic pressure limits recovery to 35 -45% F Brackish water recovery limitations – Brackish water chemistry tends to contain many sparingly soluble salts which cause scaling – Usually limits recovery to 70 -80%
Feedwater Characteristics After Pretreatment F SDI < 5. 0, preferably < 3. 0 F Al, Fe, and Mn concentrations < 0. 05 ppm F Chlorine F LSI residual < 0. 1 ppm in the brine stream slightly negative or < +1. 5 if antiscalant is used to control Ca. CO 3 scale
General Rule of Troubleshooting First Stage Problem: Fouling Last Stage Problem: Scaling

Troubleshooting F Signs of trouble – Loss of permeate flow – Increase in salt passage – Increase in DP
Calcium Carbonate Scaling F Most common precipitate F Becomes less soluble at high temperatures F Scaling caused by hardness, high p. H, high alkalinity and high recovery rates

Langelier Saturation Index Stiff and Davis Stability Index F Measure F Standard of Ca. CO 3 scaling potential ASTM procedures F LSI – Feedwater with TDS < 10, 000 mg/l – Typical limits F FILMTEC FT 30: + 1. 5 with antiscalant < 0. 0 without antiscalant F S&DSI – Feedwater with TDS >10, 000 mg/l
Calcium Carbonate Pretreatment F Antiscalant F Acid injection F Cation softening F Lime softening F Limit recovery

Sulfate Scaling F Solubility of sulfate salts increases as the ionic strength increases – Barium – Strontium – Calcium

Sulfate Pretreatment F Antiscalant F Cation injection softening F Lime softening F Limit recovery
Calcium Fluoride Scaling F Ca. F 2 solubility increases as temperature increases Pretreatment – – Antiscalant injection Cation softening Lime softening Limit recovery
Silica Scaling F Solubility is dependent on p. H, temperature, total alkalinity and Si. O 2 concentration F When supersaturated can form insoluble colloidal silica F Solubility decreases in the presence of Al or Fe. Ensure absence of Al and Fe in feed.
Silica Pretreatment F Lime softening for systems > 5, 000 m 3/day F Increase feed temperature F Increase p. H to > 8 F Reduce recovery
Fouling F Biological F Silt F Organic F Metal F Colloidal F Carbon oxide

Biological Fouling F Common in surface feedwater F Microbes adhere to membrane surface and form biofilm F Fouling caused by – Improper membrane preservation – Biological material in feedwater – Improper carbon bed maintenance
Biological Pretreatment F Chlorination – Feed must be dechlorinated to protect membranes F Shock treatment F Ozone F Granular activated carbon
Organic Fouling F Humic substances occur in concentrations between 0. 5 and 20 mg/l TOC – Pretreat when TOC exceeds 3 mg/l F High molecular mass compounds that are hydrophobic or cationic
Colloidal Fouling F Foulants of concern – Silica – Fe 2 O 3 F Determined F Consider is > 3. 0 by SDI test pretreatment if SDI
Colloidal Pretreatment F Ultrafiltration F Media to 6 F In-line filtration if SDI is > or equal filtration if SDI is very high, then use coagulation-flocculation

Silt Fouling F Caused by dirty surface water (particle laden water) with high SDI F Pretreat with media filter and/or cartridge filter F Want SDI < 5; preferably < 3

Metal Oxide Fouling F Iron F Aluminum F Manganese oxides

Iron Fouling F Caused by – Rusty well casings or piping – > 0. 1 ppm Fe in feedwater Pretreatment – Oxidation to ferric state, then filtration – Cation softening



Aluminum Fouling F Municipalities may add alum to water F Precipitation of aluminum hydroxide from acid injection Pretreatment – Minimum solubility occurs at p. H 6. 5 -6. 7 – Coagulation followed by media filtration – Concentration after pretreatment should be < 0. 05 mg/l

Manganese Oxide Fouling F Usually present as Mn. O 2 Pretreatment – Oxidation ? filtration – Concentration after pretreatment < 0. 05 mg/l

Carbon Fouling F Caused by – Inadequate flushing of carbon bed Pretreatment – Backwash and rinse carbon filter


Membrane Degradation F Damage is irreversible F Oxidation of thin-film layer – Caused by chlorine, ozone, potassium permanganate, sodium or calcium hypochlorite F Hydrolysis of the thin-film layer – Caused by high concentration of caustic (p. H >13 during cleaning)
Membrane Degradation Pretreatment F Oxidation – SBS injection – Carbon filter F Hydrolysis – Limit Na. OH concentration to 0. 1% keeping temperature < 30캜
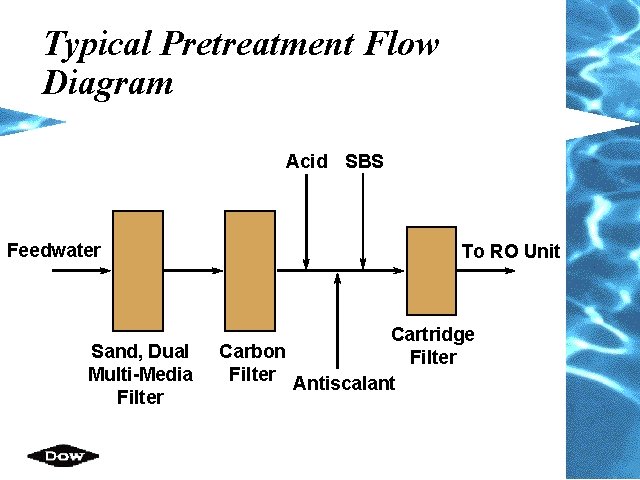
Typical Pretreatment Flow Diagram Acid SBS Feedwater Sand, Dual Multi-Media Filter To RO Unit Cartridge Carbon Filter Antiscalant
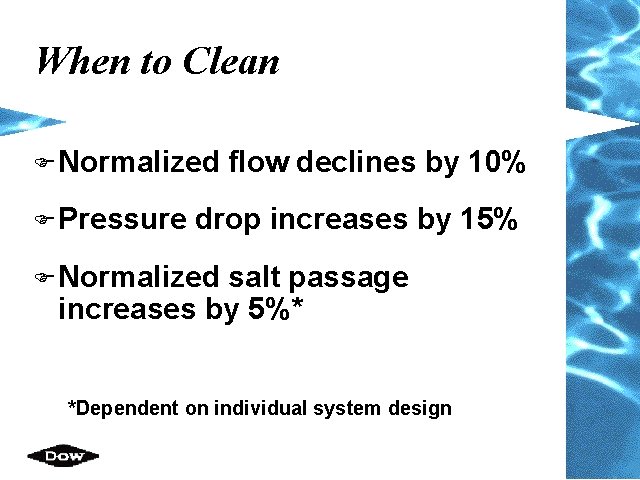
When to Clean F Normalized F Pressure flow declines by 10% drop increases by 15% F Normalized salt passage increases by 5%* *Dependent on individual system design
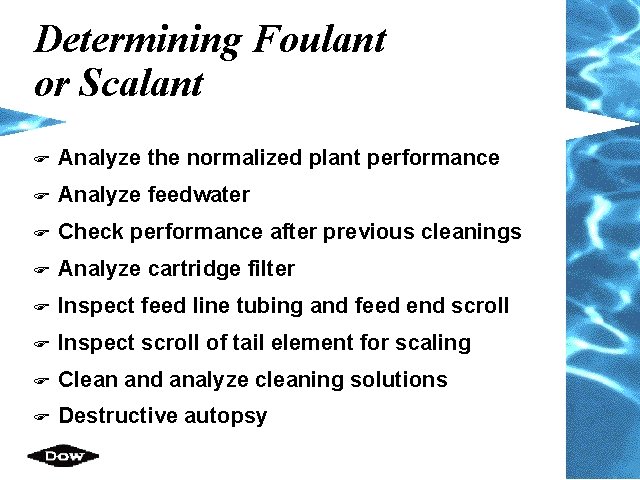
Determining Foulant or Scalant F Analyze the normalized plant performance F Analyze feedwater F Check performance after previous cleanings F Analyze cartridge filter F Inspect feed line tubing and feed end scroll F Inspect scroll of tail element for scaling F Clean and analyze cleaning solutions F Destructive autopsy
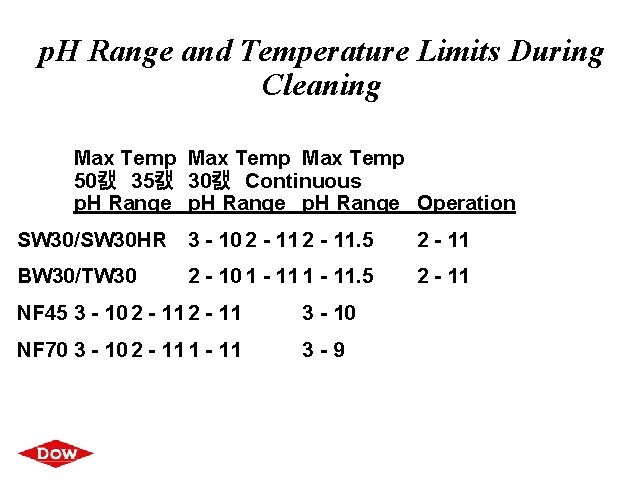
p. H Range and Temperature Limits During Cleaning Max Temp 50캜 35캜 30캜 Continuous p. H Range Operation SW 30/SW 30 HR 3 - 10 2 - 11. 5 2 - 11 BW 30/TW 30 2 - 11 2 - 10 1 - 11. 5 NF 45 3 - 10 2 - 11 3 - 10 NF 70 3 - 10 2 - 11 1 - 11 3 -9 ®

FT 30 Resistance to Cleaning Agents Agent % Concentration Resistance Hydrochloric Acid* 0. 2 Excellent EDTA*1. 0 Excellent Sodium Hydroxide* 0. 1 Excellent Nitric Acid 5. 0 Excellent Acetic Acid 5. 0 Excellent Boric Acid 5. 0 Excellent Phosphoric Acid 0. 5 Excellent Sodium Salt of Dodecylsulfate 0. 05 Excellent Sodium Lauryl Sulfate 0. 05 Excellent Sodium Hydrosulfite 1. 0 Excellent Trisodium Phosphate (TSP) 1. 0 Excellent Sodium Triphosphate (STP) 1. 0 Excellent *Most common agents used
Calcium Carbonate Scaling F Causes F Symptoms – Hardness – Heavy element – High p. H – Low permeate flow – High alkalinity – High recovery rates – Poor salt rejection – High pressure drop
Calcium Carbonate Scaling F Cleaning – 0. 2% (Wt) HCI – 2. 0% (Wt) Citric Acid – 0. 5% (Wt) H 3 PO 4 – 0. 2% (Wt) sulfamic acid and NH 2 SO 3 H
Sulfate Scaling F Causes – Exceeding solubility limits – Loss of antiscalant – High recovery rates F Symptoms – – Heavy element High pressure drop Poor salt rejection Low permeate flow

Sulfate Scaling F Cleaning – Very difficult to clean – 1. 0(Wt)% Na-EDTA and 0. 1(Wt)% Na. OH at p. H 12, 30캜 maximum F Overnight soak may be necessary
Biological Fouling F Causes – Improper membrane preservation – Biological material in feedwater – Improper carbon bed maintenance F Symptoms – – – Odor Moldy or discolored scroll end Low permeate flow Superior salt rejection High pressure drop
Biological Fouling F Cleaning (best method) – 0. 1% (Wt) Na. OH and 0. 5% - 1. 0% (Wt) Na-EDTA – At p. H 12 and < 30캜 (86캟) F Cleaning (alternate method) – 0. 1% (Wt) Na. OH and. 05% Na-DDS at p. H 12 and < 30캜 (86캟) – 1. 0% (Wt) STP and 1. 0% (Wt) Na-EDTA or 1. 0% (Wt) TSP and 1. 0% (Wt) Na-EDTA
Iron Fouling F Causes – Rusty well casings or piping – Greater than 0. 1 ppm Fe in feedwater F Symptoms – – Rust coloring on scroll end or ATD* Rusty colored reject upon start-up Low permeate flow Poor salt rejection *Anti-telescoping device

Iron Fouling F Cleaning – 1. 0% (Wt) sodium hydrosulfite (best) – 0. 5% (Wt) phosphoric acid – 0. 2% (Wt) HCl

Silt Fouling F Causes – Dirty surface waters – High SDI – Inadequate pretreatment F Symptoms – Brown or dirty scroll end – Low permeate flow/poor salt rejection (early stage) – High permeate flow/very poor salt rejection (later stage)

Silt Fouling F Cleaning – Difficult to clean – Caustic and EDTA – Detergent

Carbon Fouling F Causes – Inadequate flushing of carbon bed – Soft carbon F Symptoms – Black deposits on scroll end – Low permeate flow (early stage) – High permeate flow/very poor salt rejection (later stage)

Carbon Fouling F Cleaning – Very difficult to clean – Detergent

Chemical Attack F Causes – Incomplete dechlorination (oxidation) – Exposure to strong oxidant (i. e. permanganate) – Prolonged exposure to p. H extremes (hydrolysis) F Symptoms – Very high permeate flow – Very poor salt rejection F Damage irreversible, elements must be replaced
Permeate Backpressure Damage F Causes – Mechanical failure in system – Poor design or operation error F Symptoms – High permeate flow and very poor rejection – Wrinkles in membrane near back glue line F Damage irreversible, elements must be replaced

Cleaning Process Steps F Mix cleaning solution F Low flow pumping (low psi) F Recycle F Soak F High flow pumping (low psi) F Flush out
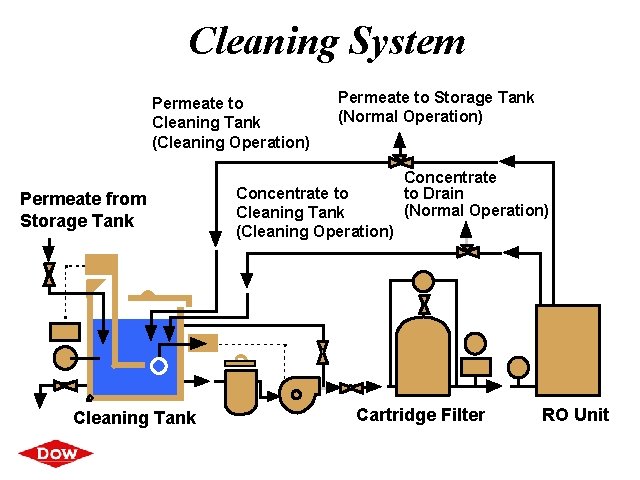
Cleaning System Permeate to Cleaning Tank (Cleaning Operation) Permeate from Storage Tank Cleaning Tank ® Permeate to Storage Tank (Normal Operation) Concentrate to Cleaning Tank (Cleaning Operation) Concentrate to Drain (Normal Operation) Cartridge Filter RO Unit

Handling & Preservation F Standard preservative solution: – 1% sodium bisulfite ? Food Grade – 18% propylene glycol

Storage Requirements F Temperature limits (-4캜 to 45캜) (22캟 to 113캟) F Inside F In cool building out of sun original packaging if possible
Storage Requirements (Cont뭗) F Dry Elements – Not affected by temperatures below -4캜 (22캟) – Storage time unlimited

Storage Requirements (Cont뭗) F Wet Elements – Inspect for biogrowth every three months – Spot check p. H of solution every three months F Represerve if it drops below 3
- Slides: 105