5 Indicators of a Chemical Change From this








- Slides: 13

5 Indicators of a Chemical Change From this lesson you will be able to determine if a chemical change has taken place (IPC 8 A & Chem 4 A).

What is a Chemical Change? • It is a change that occurs when a substance changes composition by forming one or more new substances. • The new substances/products that are formed have a different chemical formula from the original substances/reactants at the beginning.

5 Indicators of a Chemical Change • Take a look at the video of Hydrochloric Acid (HCl) reacting with Magnesium (Mg) strips. What do you observe that indicates a chemical change is taking place?

What did you observe? Bubbles Mg + 2 HCl Mg. Cl 2 + H 2 Production of Gas is an indicator of a Chemical Change. This reaction is very similar to the reactions that take place in the digestive track when Hydrochloric Acid (HCl) in your stomach aids in the digestive process. Digestion is a chemical change.

5 Indicators of a Chemical Change • Take a look at the video of a Magnesium (Mg) strip reacting with Oxygen (O 2) while burning. What do you observe that indicates a chemical change is taking place? http: //www. youtube. com/watch? v=84 v 8 y. R Pwo 5 A

What did you observe? Light 2 Mg + O 2 2 Mg. O Formation of Light is an indicator of a Chemical Change. This is the same reaction that is used to make fireworks and sparklers.

5 Indicators of a Chemical Change • Take a look at the video of two clear liquids, Lead II Nitrate Pb(NO 3)2 reacting with Potassium Iodide (KI). What do you observe that indicates a chemical change is taking place?

What did you observe? Yellow Color Change and a Precipitate (insoluble solid) Pb(NO 3)2 + 2 KI Pb. I 2 + 2 KNO 3 Permanent Color Change and Formation of a Precipitate are two indicators of a Chemical Change. Reactions like these are used in water treatment plants to form precipitates that help remove calcium, magnesium, iron, and manganese ions from the water.

5 Indicators of a Chemical Change • Take a look at the video of two solids, Barium Hydroxide Octahydrate reacting with Ammonium Thiocyanate. What do you observe that indicates a chemical change is taking place?

What did you observe? A Temperature Change Ba(OH)2. 8 H 2 O + 2 NH 4 SCN --> Ba(SCN)2 + 10 H 2 O + 2 NH 3 Temperature Change is an indicator of a Chemical Change. Endothermic Reactions = absorb heat and feel cold Exothermic Reactions = release heat and feel hot

Let’s Review The 5 Indicators of a Chemical Change are… 1. Production of Gas 2. Production of Light 3. Permanent Color Change 4. Formation of a Precipitate 5. Temperature Change

Test Your Knowledge • Go to the Live Chem website below: • http: //www. chem. ox. ac. uk/vrchemistry/Live. Chem/transitionmetals_content. html • While in the Virtual Chemistry Lab, perform the following 5 experiments and record whether they demonstrate a chemical change. Then choose 5 other experimental mixes of your own and record whether they demonstrate a chemical change. Use the 5 Indicators of a Chemical Change to support your findings for all 10 experiments conducted. • Click on the next slide for the Lab Write-up.

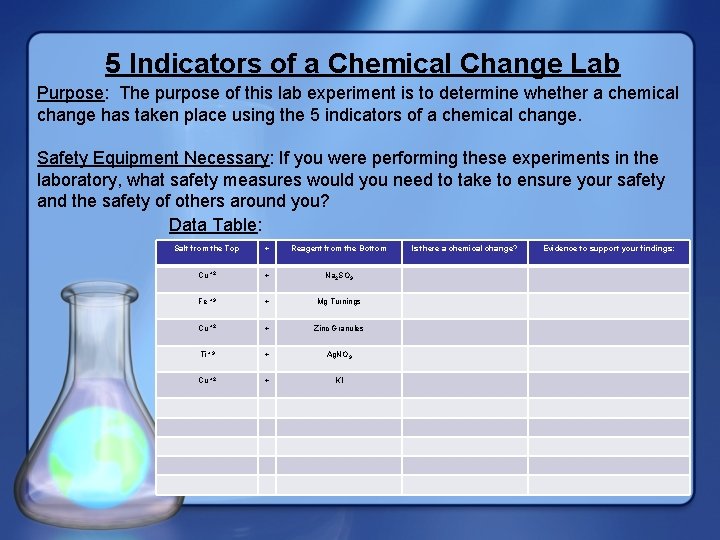
5 Indicators of a Chemical Change Lab Purpose: The purpose of this lab experiment is to determine whether a chemical change has taken place using the 5 indicators of a chemical change. Safety Equipment Necessary: If you were performing these experiments in the laboratory, what safety measures would you need to take to ensure your safety and the safety of others around you? Data Table: Salt from the Top + Reagent from the Bottom Cu +2 + Na 2 SO 3 Fe +3 + Mg Turnings Cu +2 + Zinc Granules Ti +3 + Ag. NO 3 Cu +2 + KI Is there a chemical change? Evidence to support your findings:
 What are 5 indicators of a chemical reaction
What are 5 indicators of a chemical reaction 5 indicators of a chemical reaction
5 indicators of a chemical reaction Is painting a wall a physical change
Is painting a wall a physical change Changes in matter
Changes in matter Whats the difference between a chemical and physical change
Whats the difference between a chemical and physical change Which is an example of a physical change
Which is an example of a physical change Spare change physical versus chemical change
Spare change physical versus chemical change Whats the difference between a chemical and physical change
Whats the difference between a chemical and physical change How does a physical change differ from a chemical change
How does a physical change differ from a chemical change Study jams physical and chemical changes
Study jams physical and chemical changes Chopping wood physical or chemical
Chopping wood physical or chemical 5 indicators of a chemical reaction
5 indicators of a chemical reaction Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds