Matter and Energy Matter anything that occupies space















- Slides: 78

Matter and Energy § Matter – anything that occupies space and has mass (weight) § Energy – the ability to do work § Chemical § Electrical § Mechanical § Radiant Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

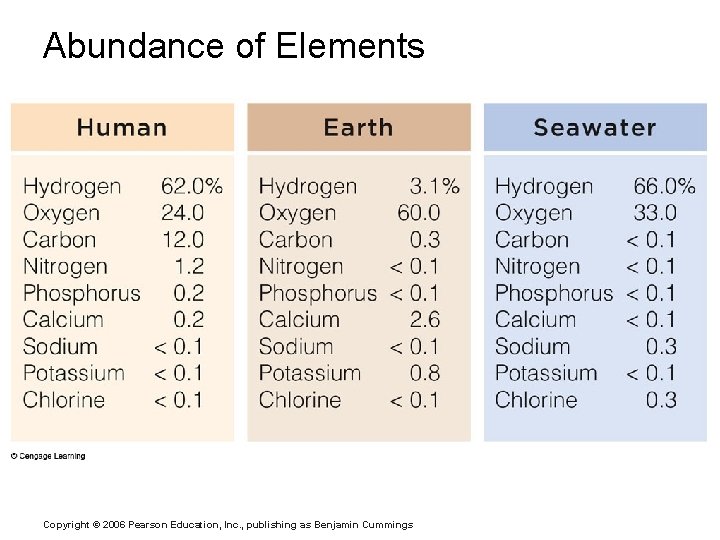
Composition of Matter § Elements § Fundamental units of matter § 96% of the body is made from four elements § Carbon (C) § Oxygen (O) § Hydrogen (H) § Nitrogen (N) § Atoms § Building blocks of elements Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Abundance of Elements Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

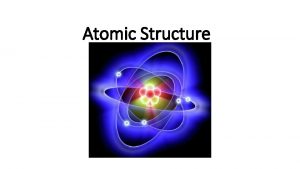
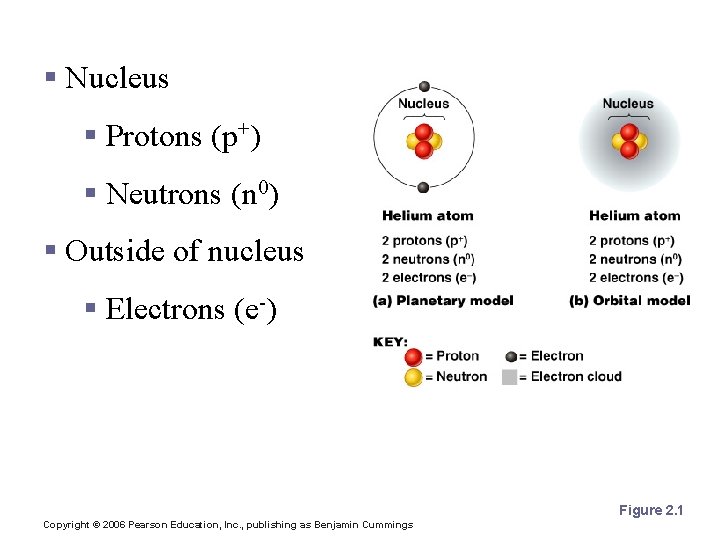
Atomic Structure § Nucleus § Protons (p+) § Neutrons (n 0) § Outside of nucleus § Electrons (e-) Figure 2. 1 Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Identifying Elements § Atomic number § Equal to the number of protons that the atoms contain § Atomic mass number § Sum of the protons and neutrons Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

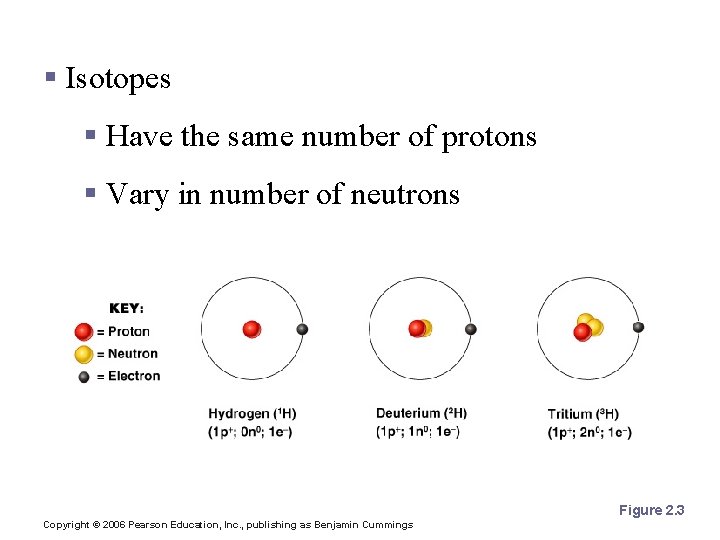
Isotopes and Atomic Weight § Isotopes § Have the same number of protons § Vary in number of neutrons Figure 2. 3 Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Isotopes and Atomic Weight § Atomic weight § Close to mass number of most abundant isotope § Atomic weight reflects natural isotope variation Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Radioactivity § Radioisotope § Heavy isotope § Tends to be unstable § Decomposes to more stable isotope § Radioactivity § Process of spontaneous atomic decay Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Molecules and Compounds § Molecule – two or more like atoms combined chemically § Compound – two or more different atoms combined chemically Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Chemical Reactions § Atoms are united by chemical bonds § Atoms dissociate from other atoms when chemical bonds are broken Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Electrons and Bonding § Electrons occupy energy levels called electron shells § Electrons closest to the nucleus are most strongly attracted § Each shell has distinct properties § Number of electrons has an upper limit § Shells closest to nucleus fill first Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Electrons and Bonding § Bonding involves interactions between electrons in the outer shell (valence shell) § Full valence shells do not form bonds Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

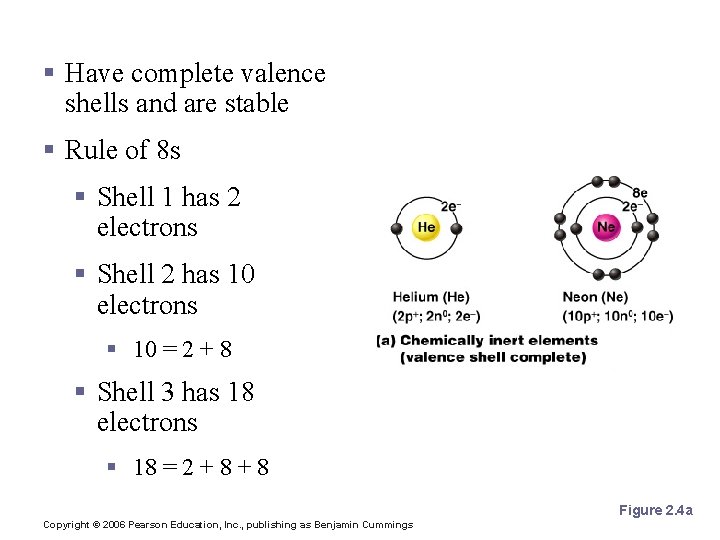
Inert Elements § Have complete valence shells and are stable § Rule of 8 s § Shell 1 has 2 electrons § Shell 2 has 10 electrons § 10 = 2 + 8 § Shell 3 has 18 electrons § 18 = 2 + 8 Figure 2. 4 a Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

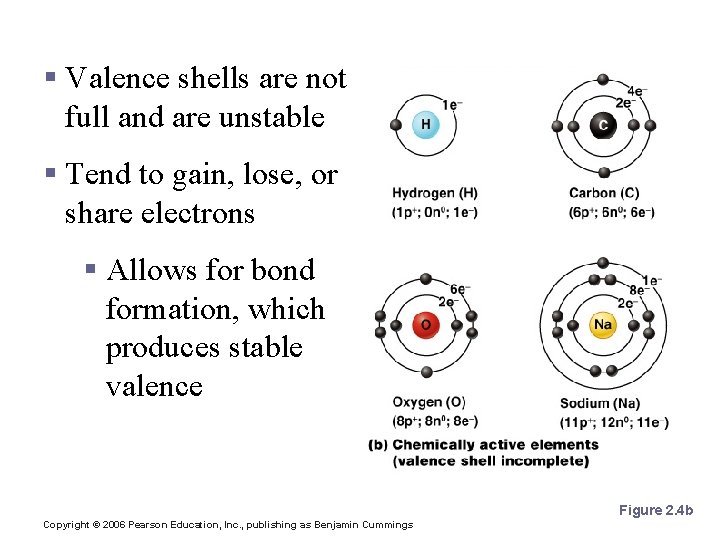
Reactive Elements § Valence shells are not full and are unstable § Tend to gain, lose, or share electrons § Allows for bond formation, which produces stable valence Figure 2. 4 b Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

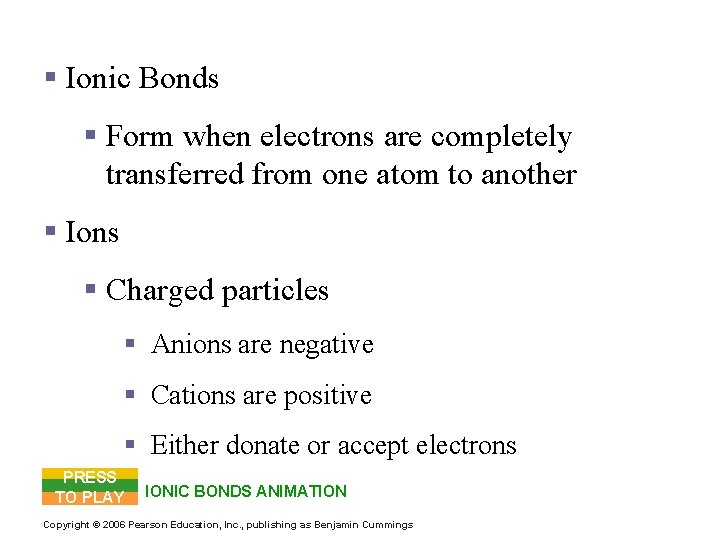
Chemical Bonds § Ionic Bonds § Form when electrons are completely transferred from one atom to another § Ions § Charged particles § Anions are negative § Cations are positive § Either donate or accept electrons PRESS TO PLAY IONIC BONDS ANIMATION Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

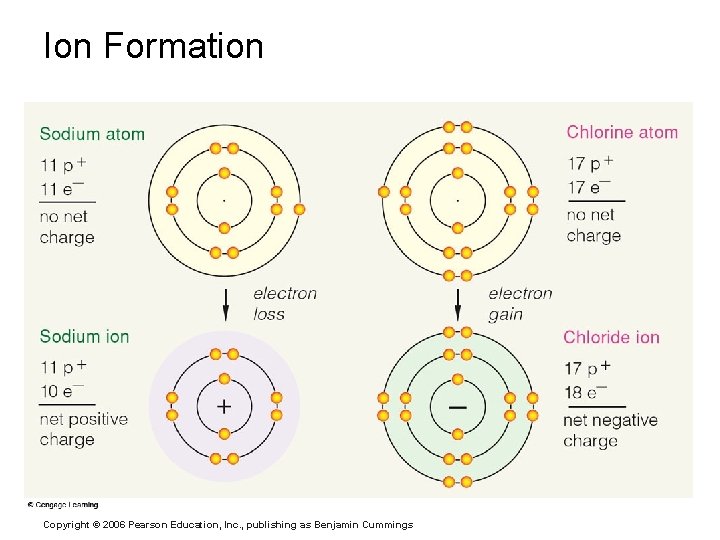
Ion Formation Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

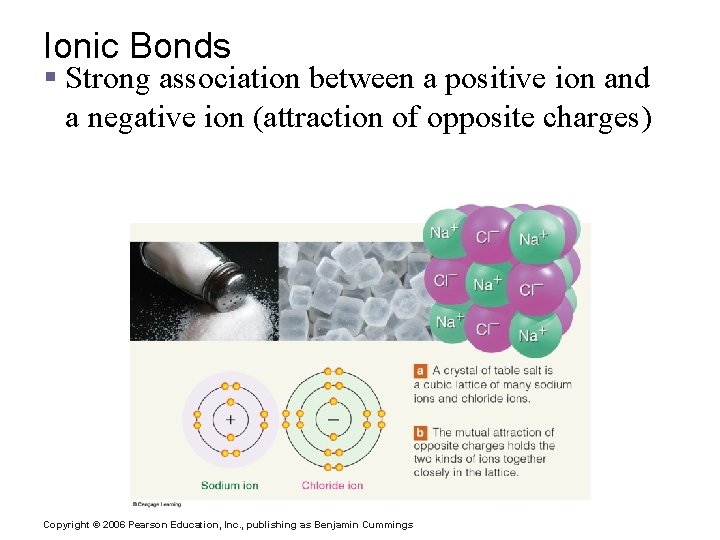
Ionic Bonds § Strong association between a positive ion and a negative ion (attraction of opposite charges) Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

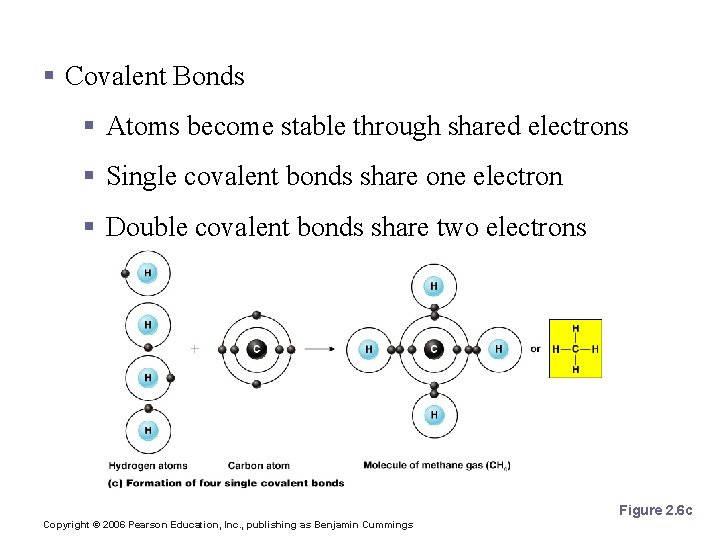
Chemical Bonds § Covalent Bonds § Atoms become stable through shared electrons § Single covalent bonds share one electron § Double covalent bonds share two electrons Figure 2. 6 c Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

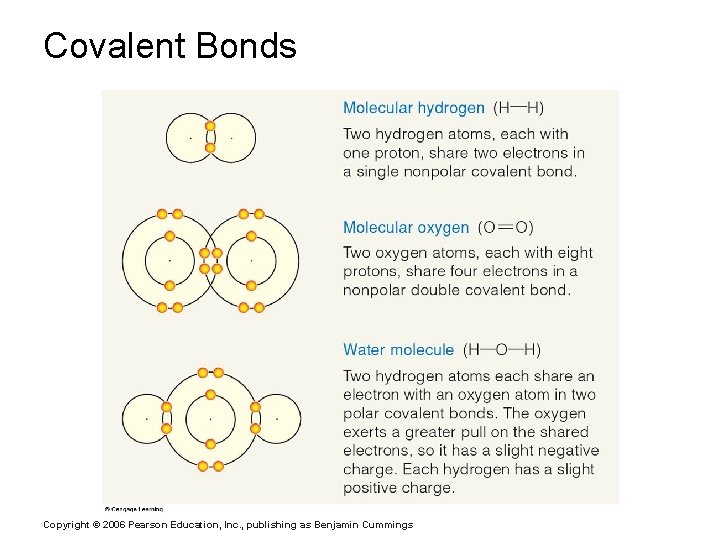
Covalent Bonds Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

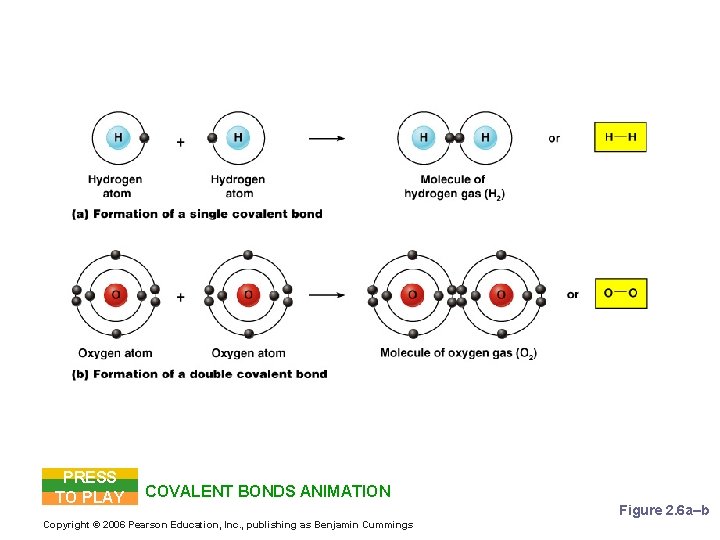
Examples of Covalent Bonds PRESS TO PLAY COVALENT BONDS ANIMATION Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings Figure 2. 6 a–b

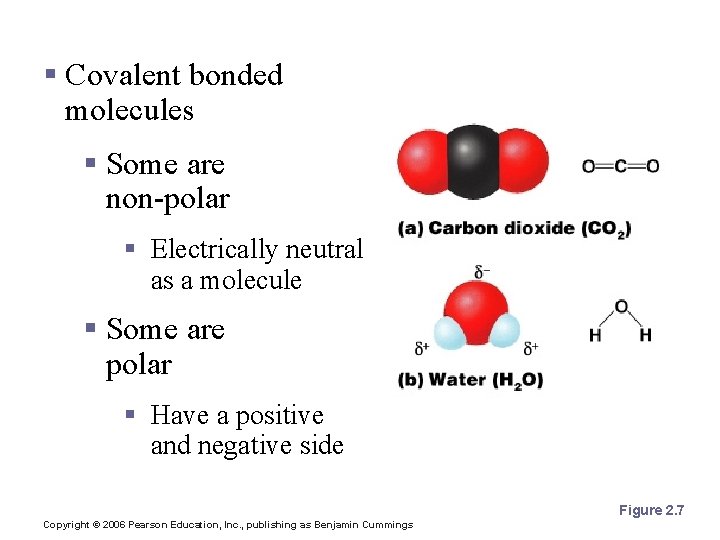
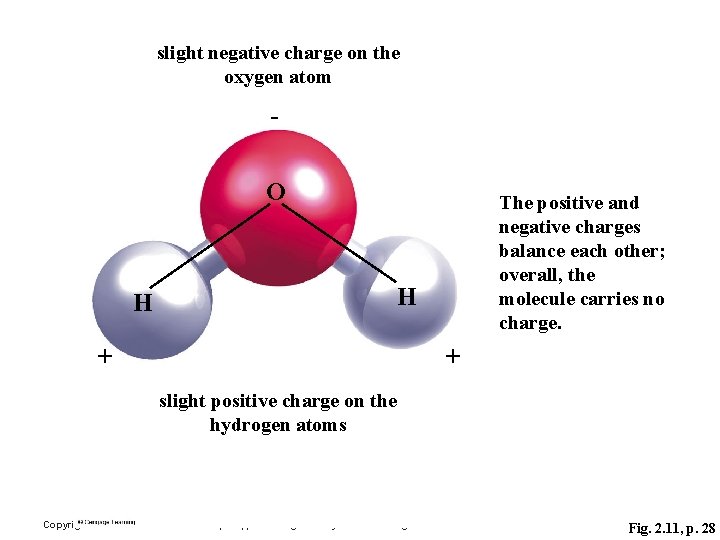
Polarity § Covalent bonded molecules § Some are non-polar § Electrically neutral as a molecule § Some are polar § Have a positive and negative side Figure 2. 7 Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

slight negative charge on the oxygen atom - O The positive and negative charges balance each other; overall, the molecule carries no charge. H H + + slight positive charge on the hydrogen atoms Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings Fig. 2. 11, p. 28

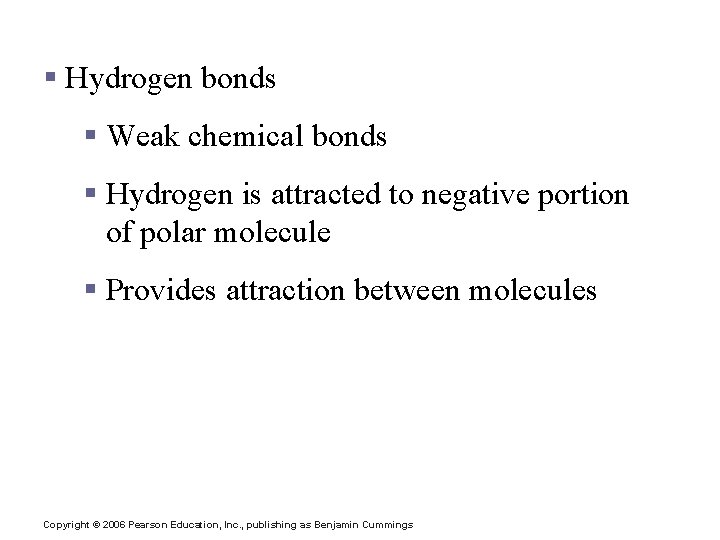
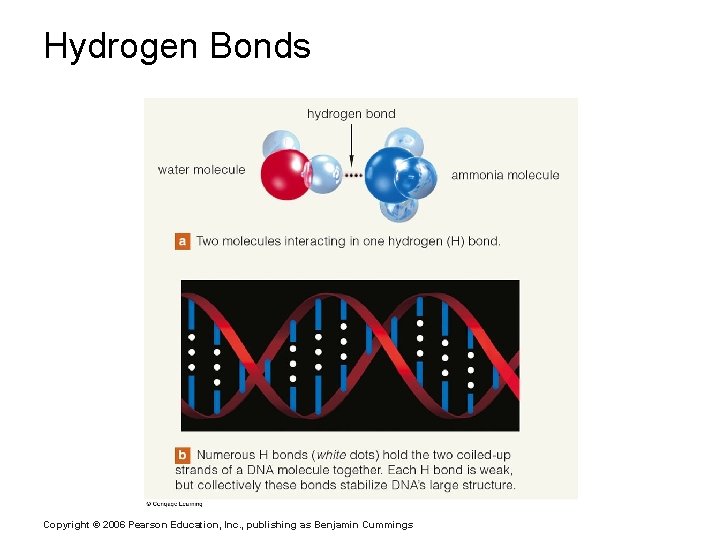
Chemical Bonds § Hydrogen bonds § Weak chemical bonds § Hydrogen is attracted to negative portion of polar molecule § Provides attraction between molecules Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Hydrogen Bonds Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

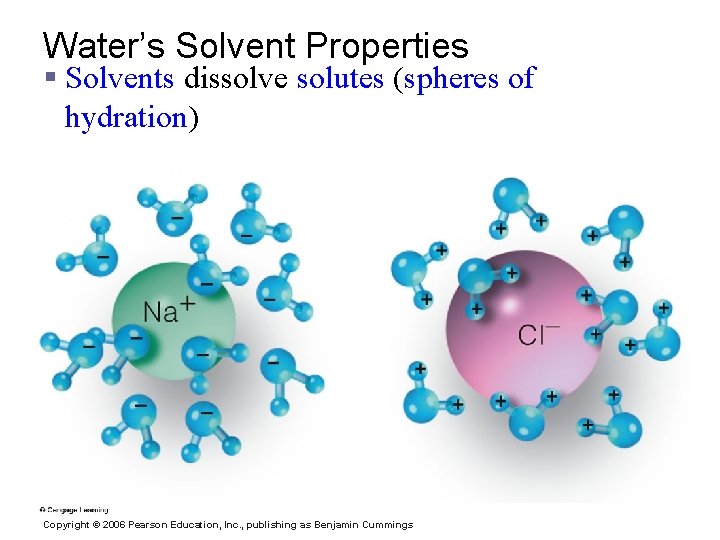
Water’s Solvent Properties § Solvents dissolve solutes (spheres of hydration) Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

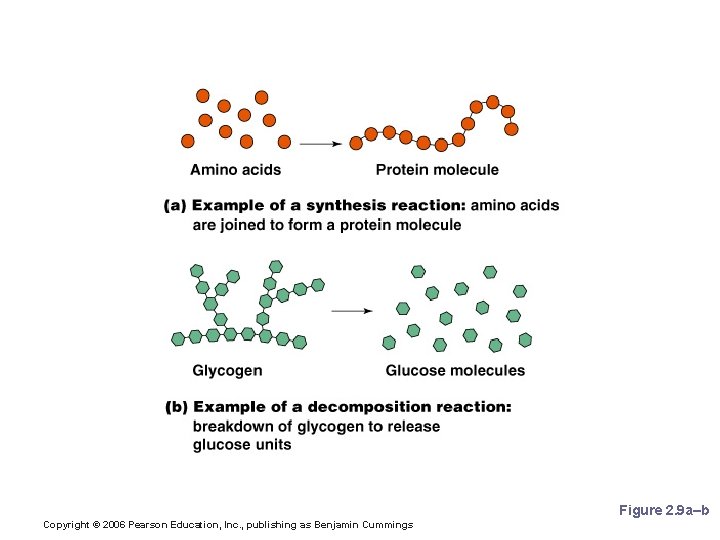
Patterns of Chemical Reactions § Synthesis reaction (A+B AB) § Atoms or molecules combine § Energy is absorbed for bond formation § Decomposition reaction (AB A+B) § Molecule is broken down § Chemical energy is released Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Synthesis and Decomposition Reactions Figure 2. 9 a–b Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

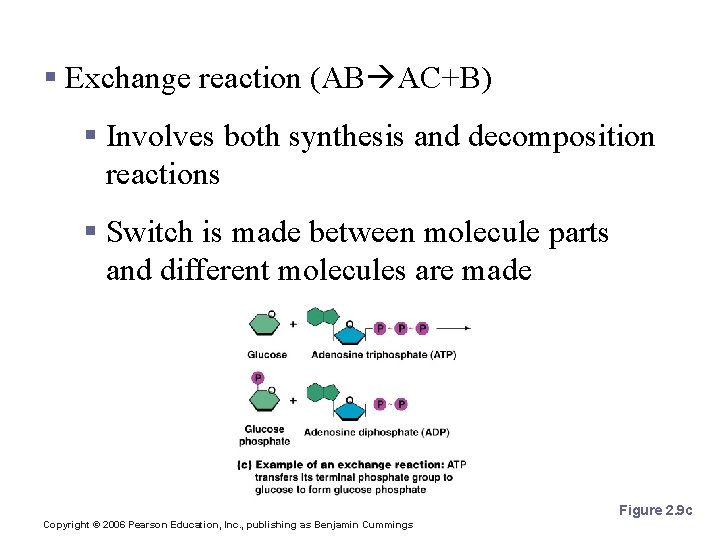
Patterns of Chemical Reactions § Exchange reaction (AB AC+B) § Involves both synthesis and decomposition reactions § Switch is made between molecule parts and different molecules are made Figure 2. 9 c Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Biochemistry: Essentials for Life § Organic compounds § Contain carbon § Most are covalently bonded § Example: C 6 H 12 O 6 (glucose) § Inorganic compounds § Lack carbon § Tend to be simpler compounds § Example: H 2 O (water) Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Important Inorganic Compounds § Water § Most abundant inorganic compounds § Vital properties § High heat capacity § Polarity/solvent properties § Chemical reactivity § Cushioning Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Important Inorganic Compounds § Salts § Easily dissociate into ions in the presence of water § Vital to many body functions § Include electrolytes which conduct electrical currents Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Important Inorganic Compounds § Acids § Can release detectable hydrogen ions § Bases § Proton acceptors § Neutralization reaction § Acids and bases react to form water and a salt Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

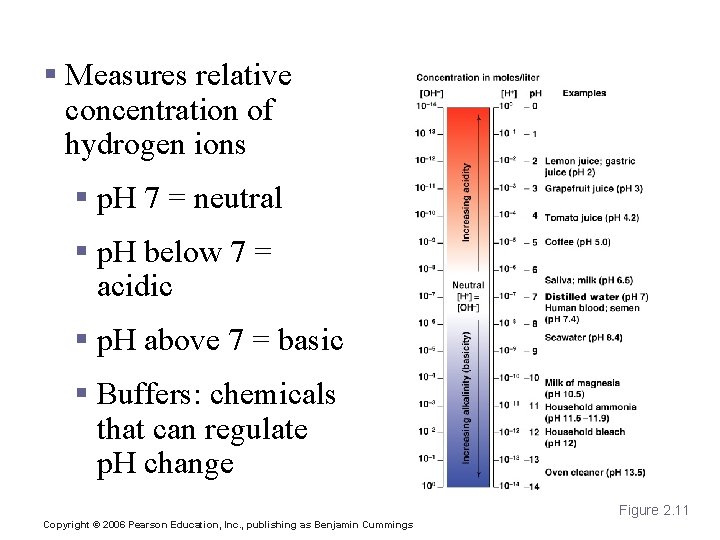
p. H § Measures relative concentration of hydrogen ions § p. H 7 = neutral § p. H below 7 = acidic § p. H above 7 = basic § Buffers: chemicals that can regulate p. H change Figure 2. 11 Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

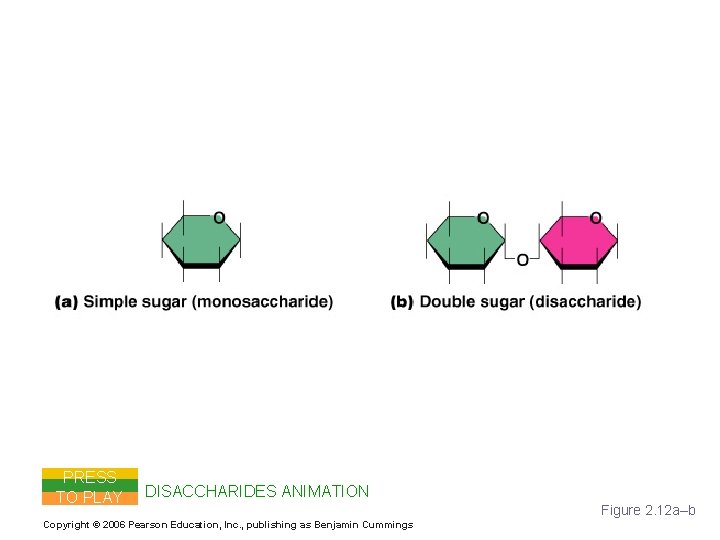
Important Organic Compounds § Carbohydrates § Contain carbon, hydrogen, and oxygen § Include sugars and starches § Classified according to size § Monosaccharides – simple sugars § Disaccharides – two simple sugars joined by dehydration synthesis § Polysaccharides – long branching chains of linked simple sugars Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

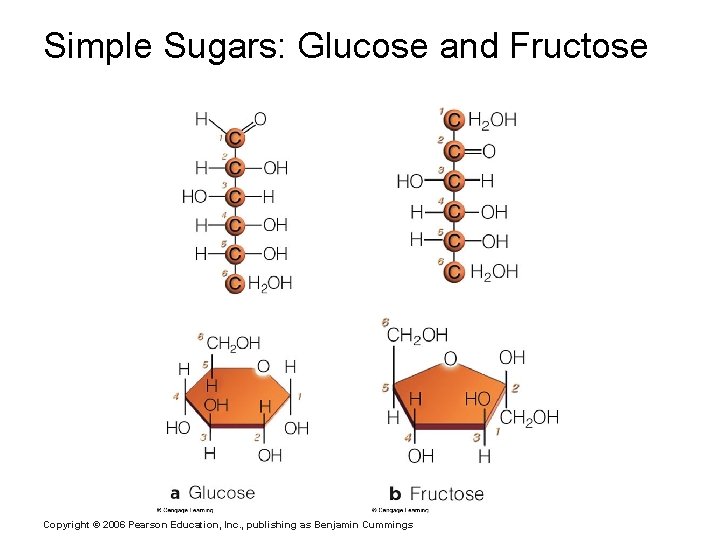
Simple Sugars: Glucose and Fructose Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Carbohydrates PRESS TO PLAY DISACCHARIDES ANIMATION Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings Figure 2. 12 a–b

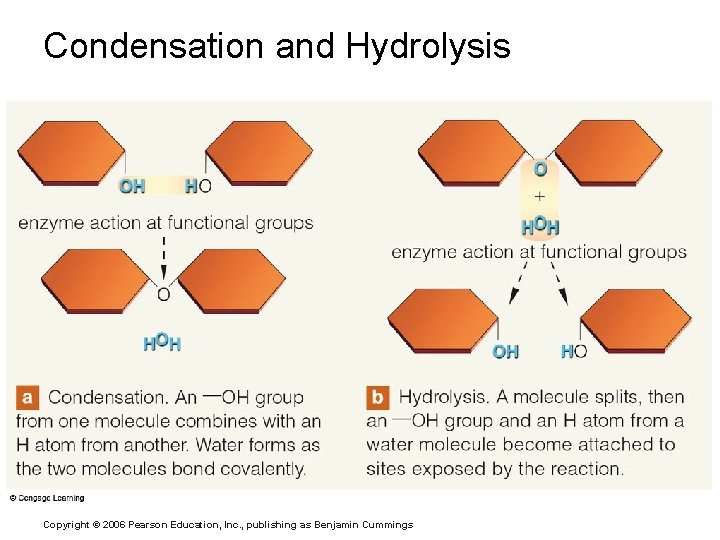
Condensation and Hydrolysis Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

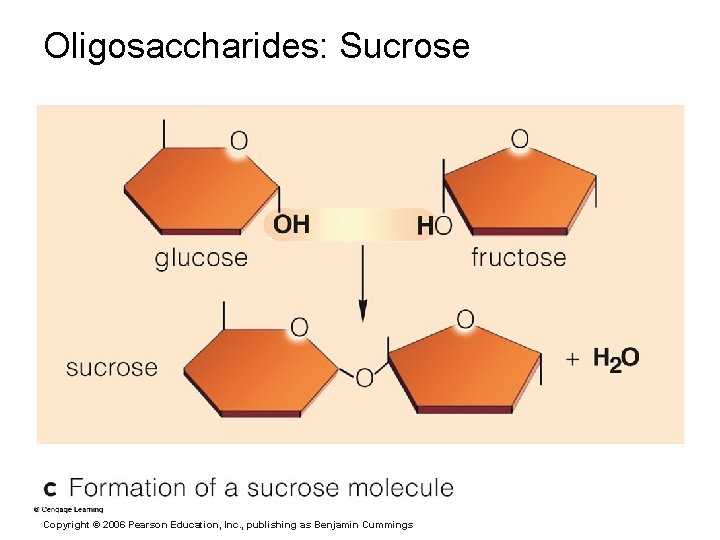
Oligosaccharides: Sucrose Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

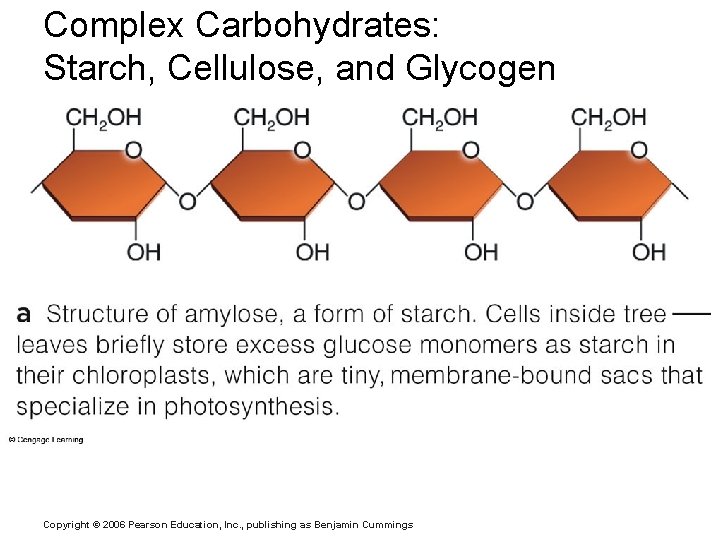
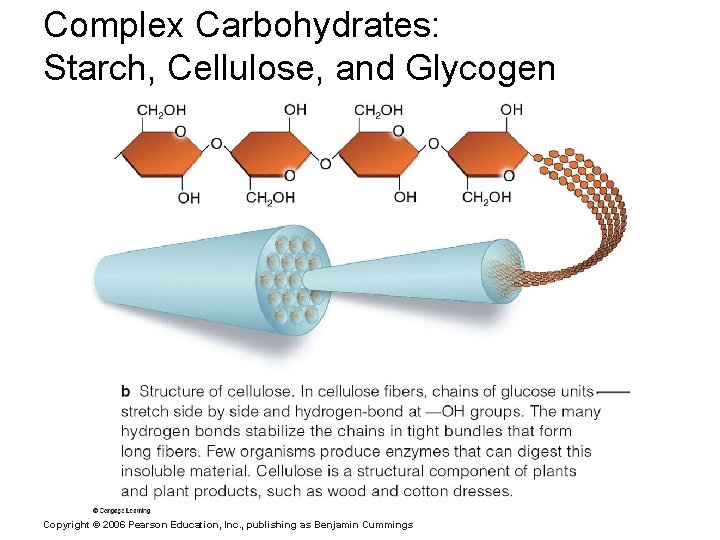
Complex Carbohydrates: Starch, Cellulose, and Glycogen Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Complex Carbohydrates: Starch, Cellulose, and Glycogen Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

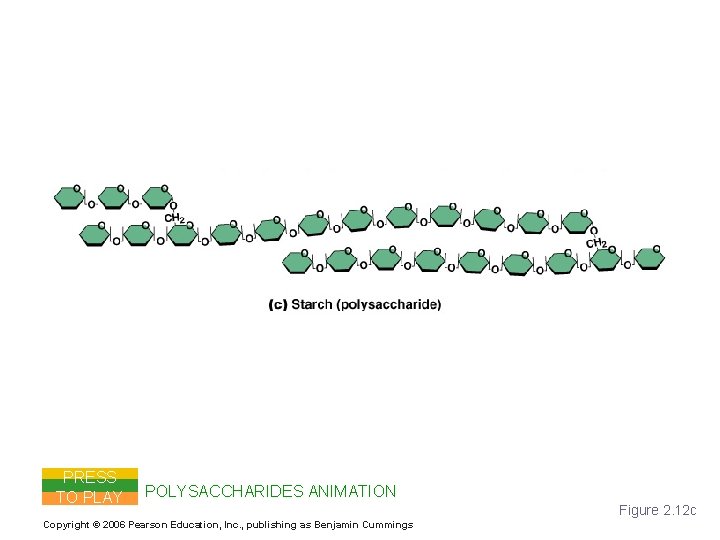
Carbohydrates PRESS TO PLAY POLYSACCHARIDES ANIMATION Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings Figure 2. 12 c

Important Organic Compounds § Lipids § Contain carbon, hydrogen, and oxygen § Carbon and hydrogen outnumber oxygen § Insoluble in water PRESS TO PLAY LIPIDS ANIMATION Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

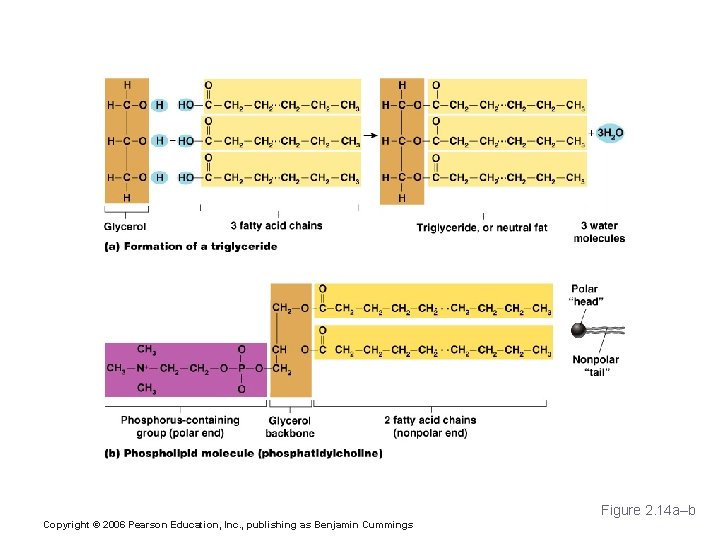
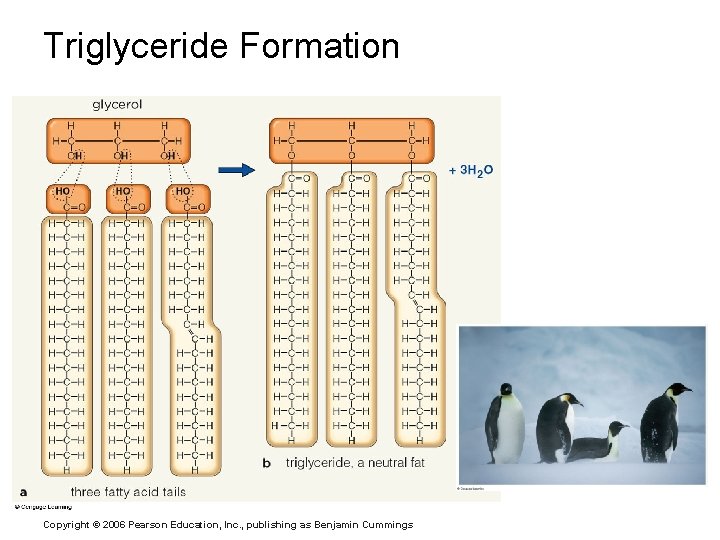
Lipids § Common lipids in the human body § Neutral fats (triglycerides) § Found in fat deposits § Composed of fatty acids and glycerol § Source of stored energy Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Lipids § Common lipids in the human body (continued) § Phospholipids § Form cell membranes § Steroids § Include cholesterol, bile salts, vitamin D, and some hormones Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

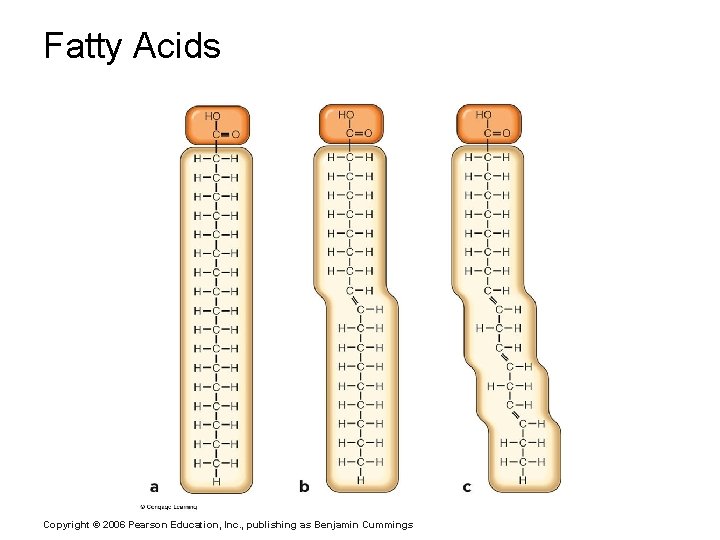
Fatty Acids Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

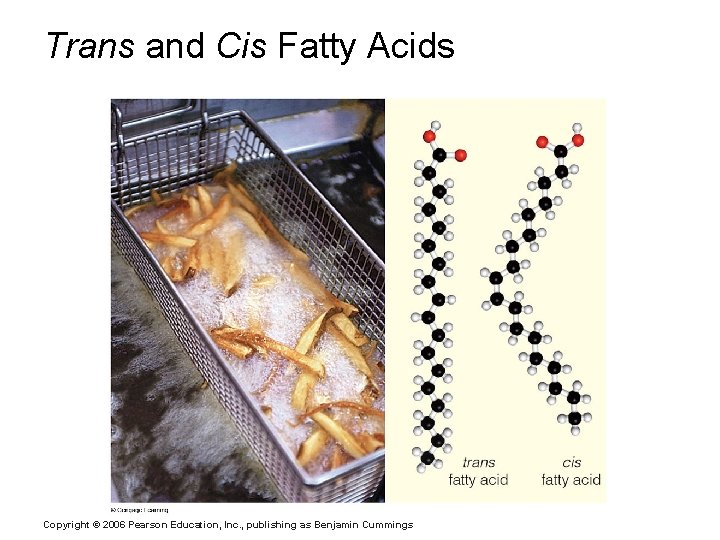
Trans and Cis Fatty Acids Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Lipids Figure 2. 14 a–b Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Triglyceride Formation Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

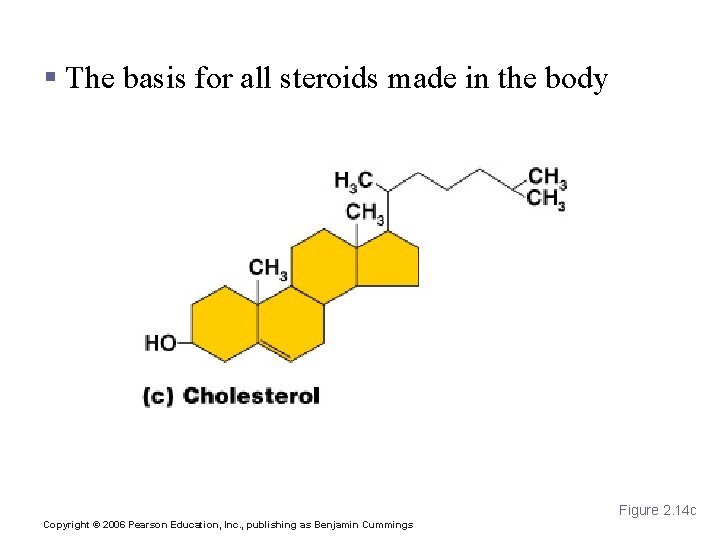
Cholesterol § The basis for all steroids made in the body Figure 2. 14 c Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Important Organic Compounds § Proteins § Made of amino acids § Contain carbon, oxygen, hydrogen, nitrogen, and sometimes sulfur Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

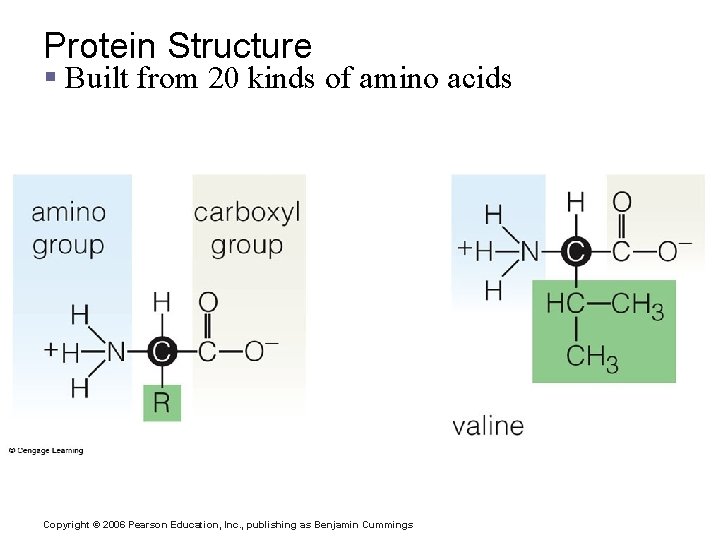
Protein Structure § Built from 20 kinds of amino acids Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Proteins § Account for over half of the body’s organic matter § Provides for construction materials for body tissues § Plays a vital role in cell function § Act as enzymes, hormones, and antibodies PRESS TO PLAY CHEMISTRY OF LIFE© PROTEINS: ENZYME ANIMATION Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

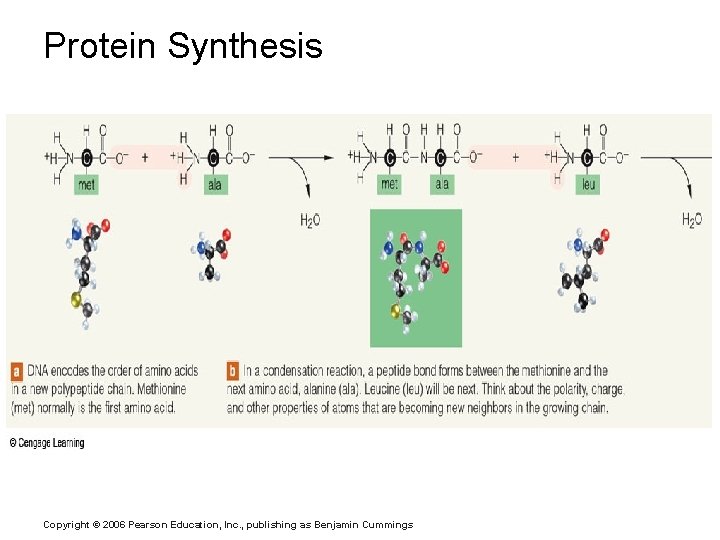
Protein Synthesis Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

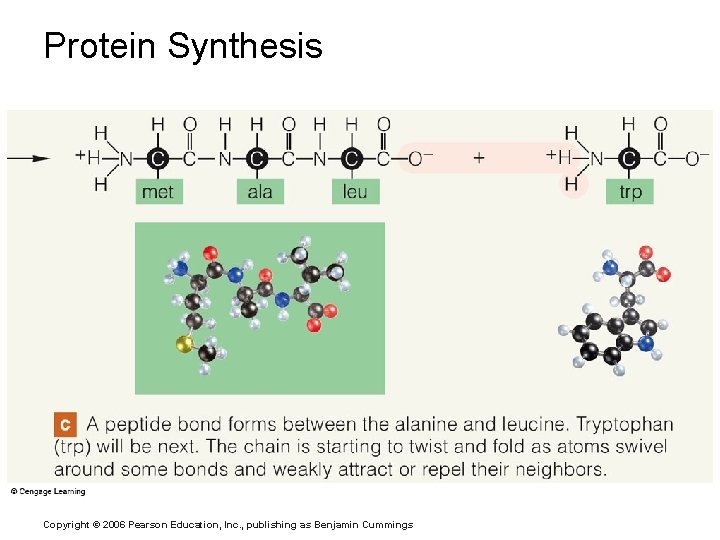
Protein Synthesis Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

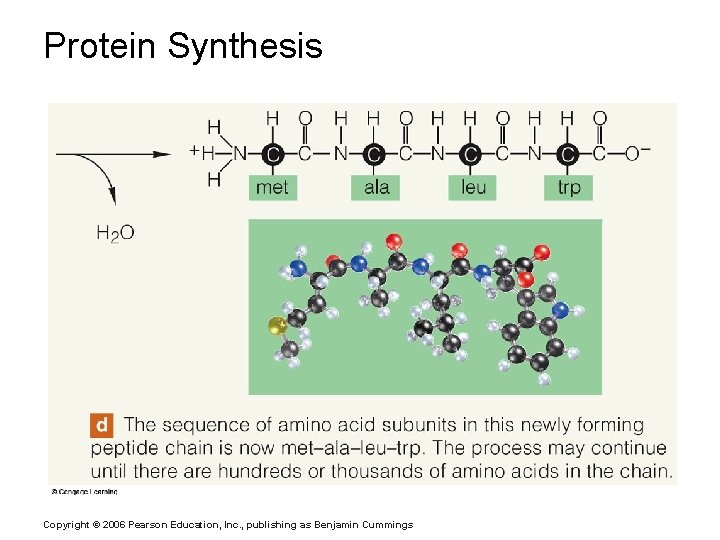
Protein Synthesis Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

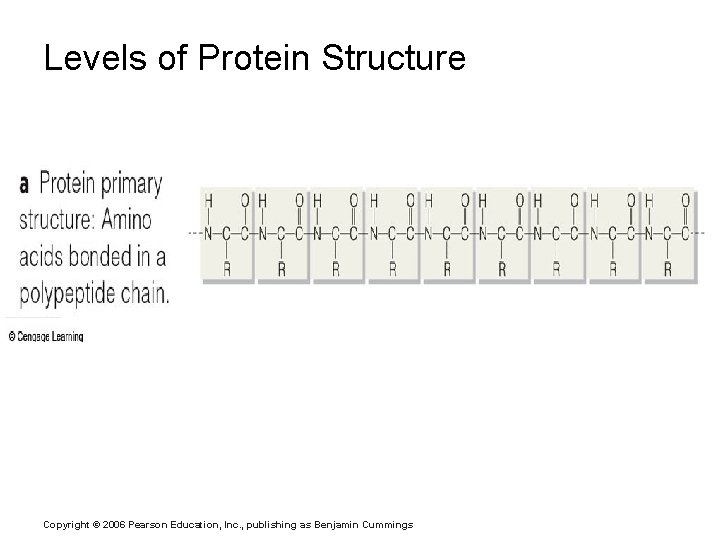
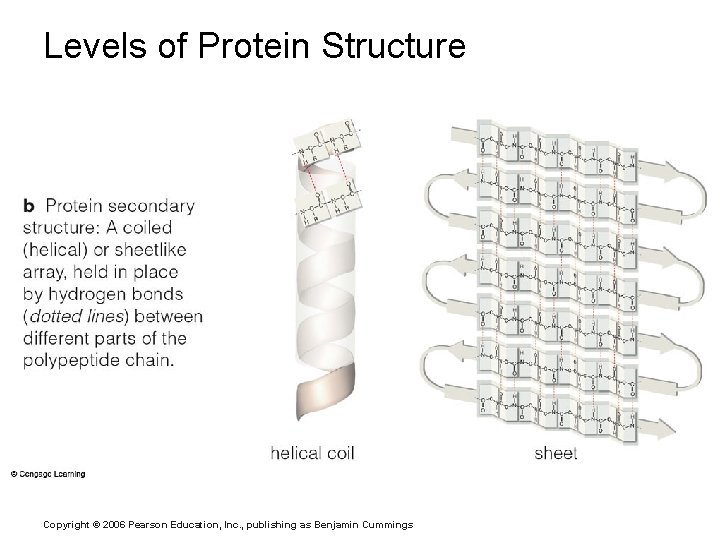
Levels of Protein Structure Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

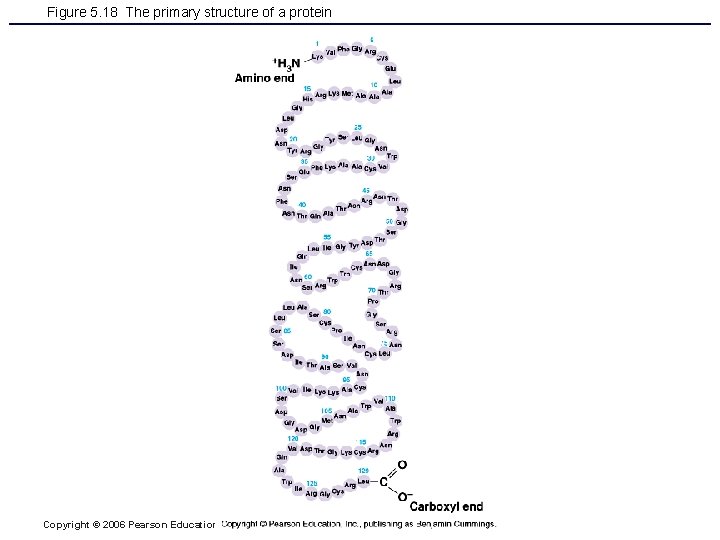
Figure 5. 18 The primary structure of a protein Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

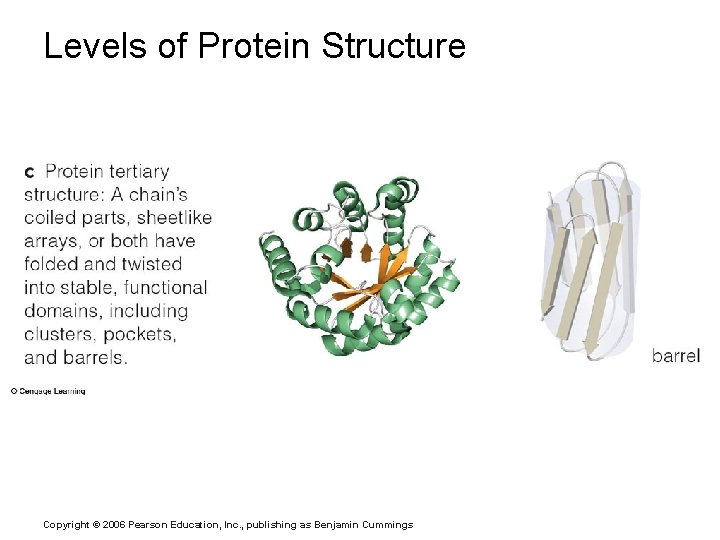
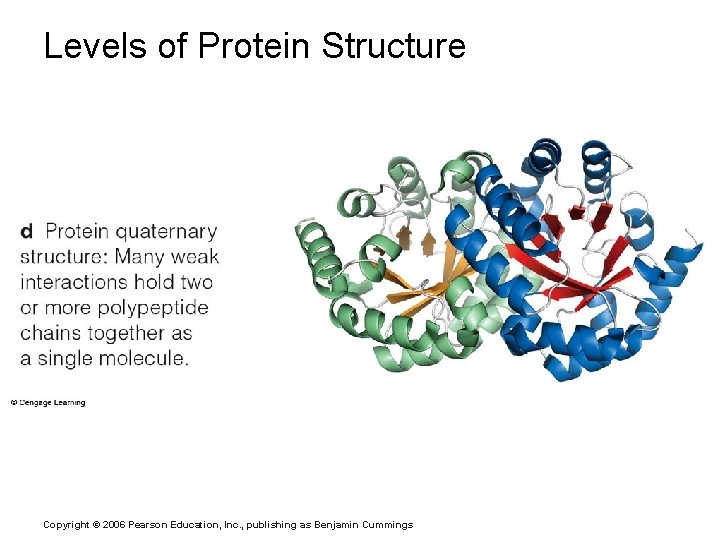
Levels of Protein Structure Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Levels of Protein Structure Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

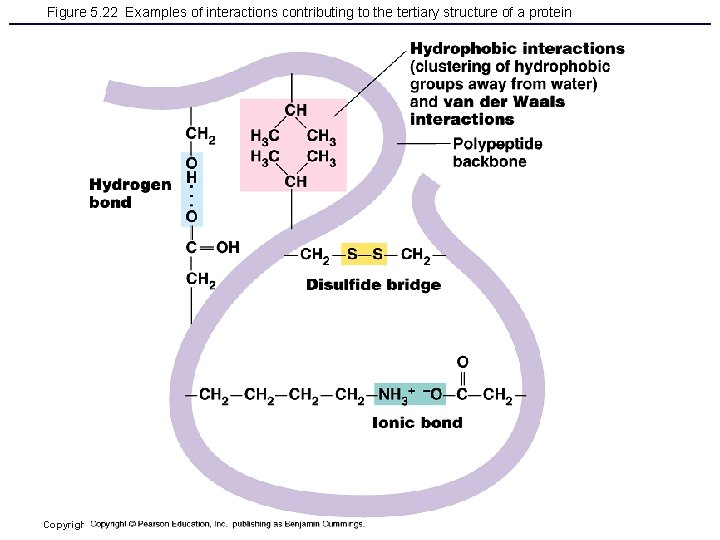
Figure 5. 22 Examples of interactions contributing to the tertiary structure of a protein Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Levels of Protein Structure Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

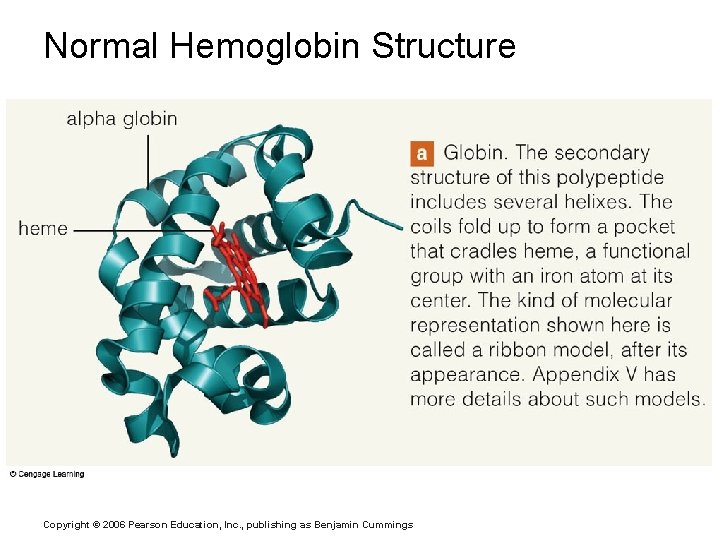
Normal Hemoglobin Structure Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

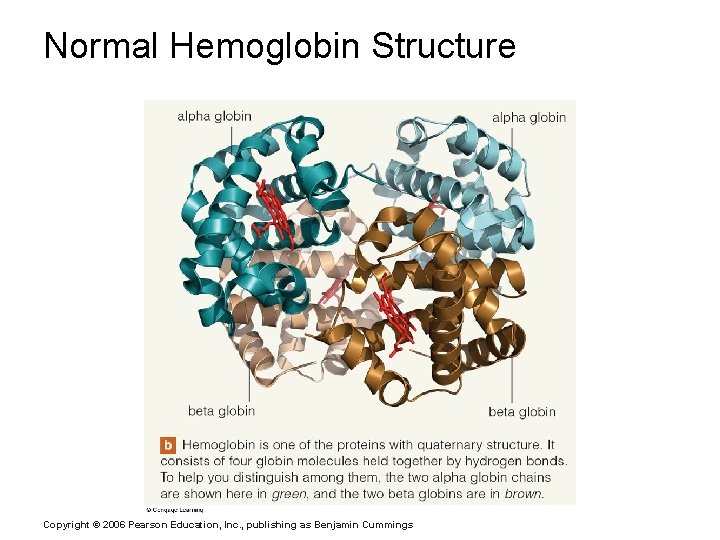
Normal Hemoglobin Structure Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

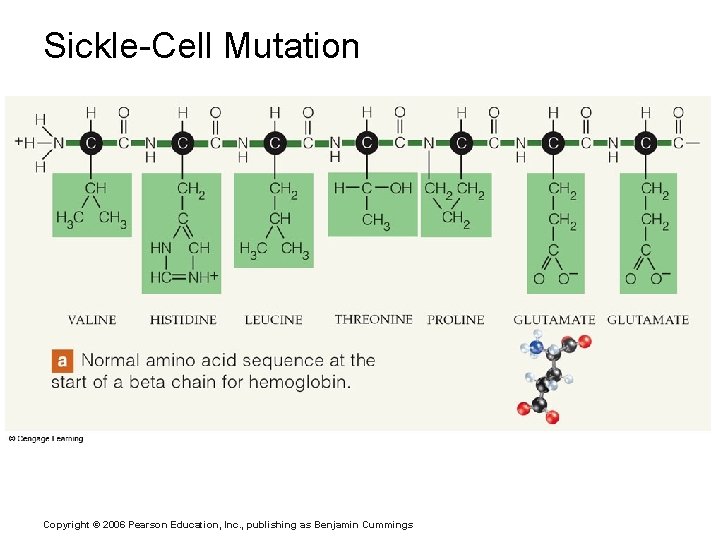
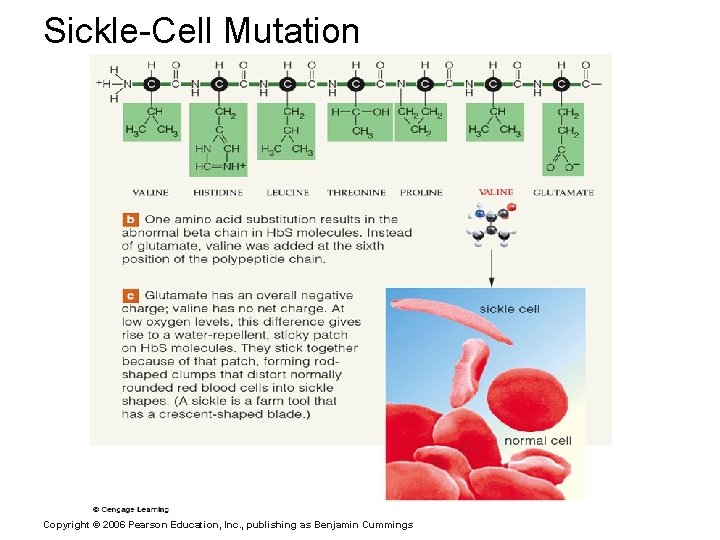
Sickle-Cell Mutation Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Sickle-Cell Mutation Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

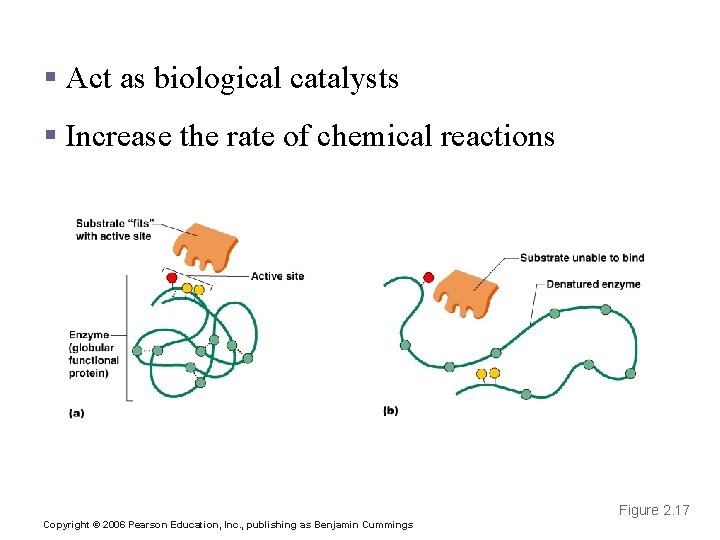
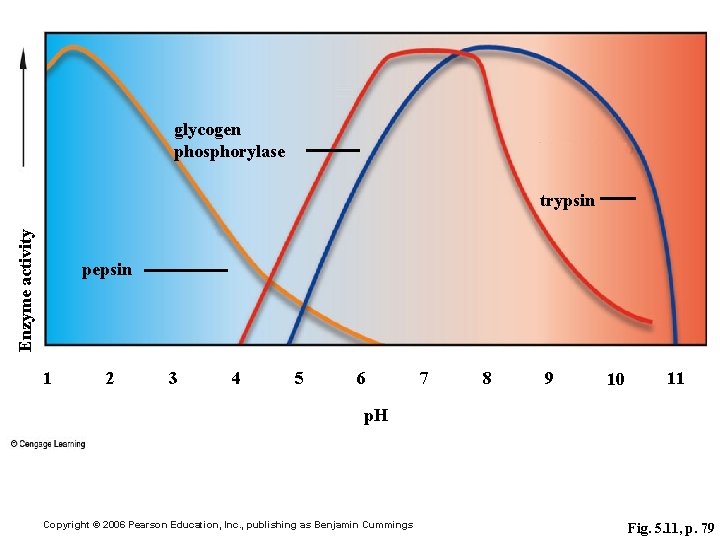
Enzymes § Act as biological catalysts § Increase the rate of chemical reactions Figure 2. 17 Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

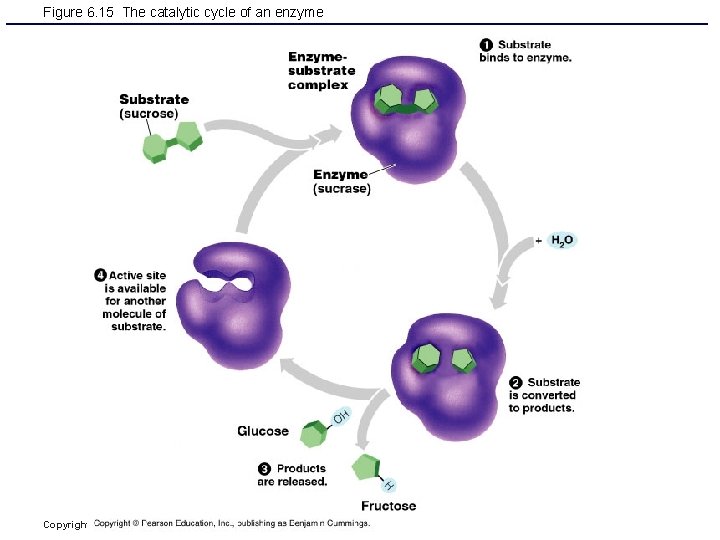
Figure 6. 15 The catalytic cycle of an enzyme Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

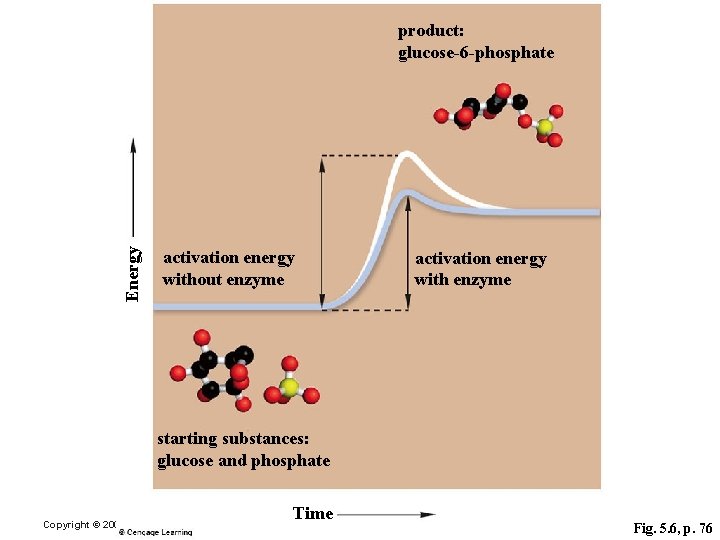
Energy product: glucose-6 -phosphate activation energy without enzyme activation energy with enzyme starting substances: glucose and phosphate Time Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings Fig. 5. 6, p. 76

glycogen phosphorylase Enzyme activity trypsin pepsin 1 2 3 4 5 6 7 8 9 10 11 p. H Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings Fig. 5. 11, p. 79

Important Organic Compounds § Nucleic Acids § Provide blueprint of life § Nucleotide bases § A = Adenine § G = Guanine § C = Cytosine § T = Thymine § U = Uracil § Make DNA and RNA Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

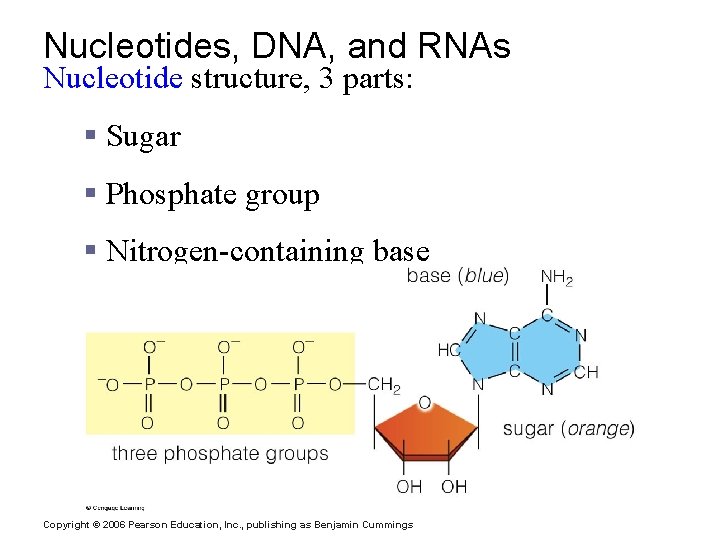
Nucleotides, DNA, and RNAs Nucleotide structure, 3 parts: § Sugar § Phosphate group § Nitrogen-containing base Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

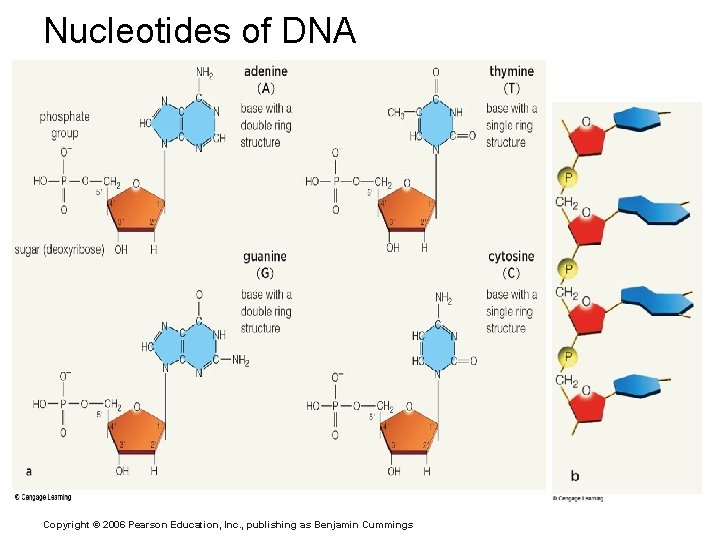
Nucleotides of DNA Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

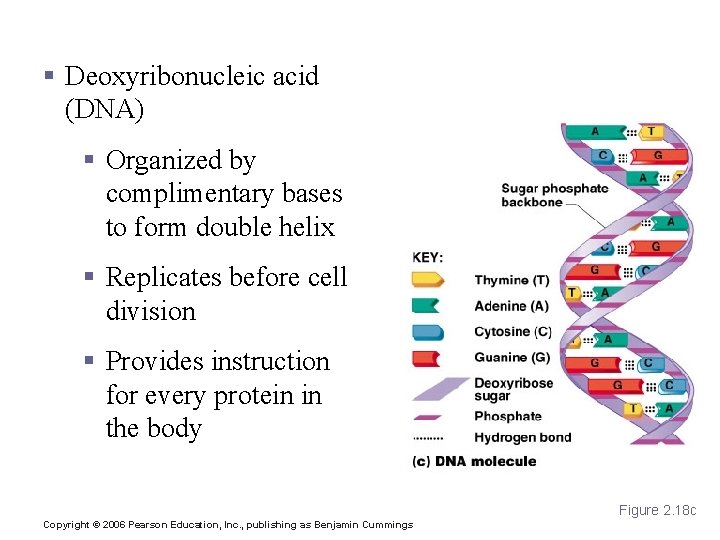
Nucleic Acids § Deoxyribonucleic acid (DNA) § Organized by complimentary bases to form double helix § Replicates before cell division § Provides instruction for every protein in the body Figure 2. 18 c Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

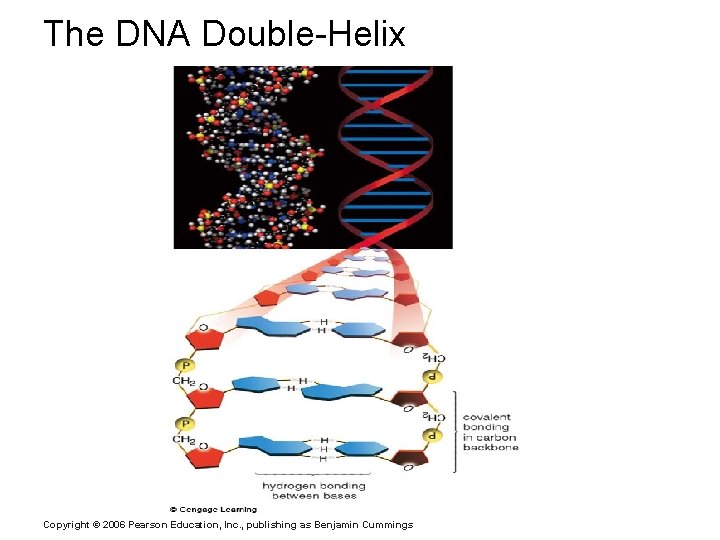
The DNA Double-Helix Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

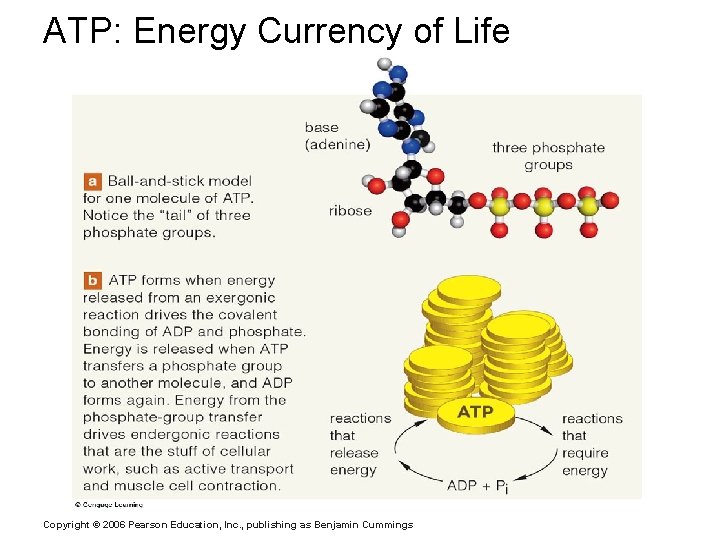
Important Organic Compounds § Adenosine triphosphate (ATP) § Chemical energy used by all cells § Energy is released by breaking high energy phosphate bond § ATP is replenished by oxidation of food fuels Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

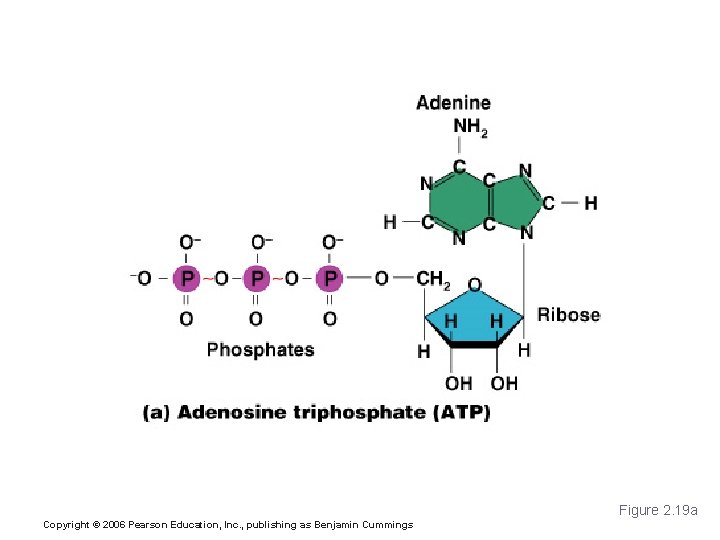
Adenosine Triphosphate (ATP) Figure 2. 19 a Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

ATP: Energy Currency of Life Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

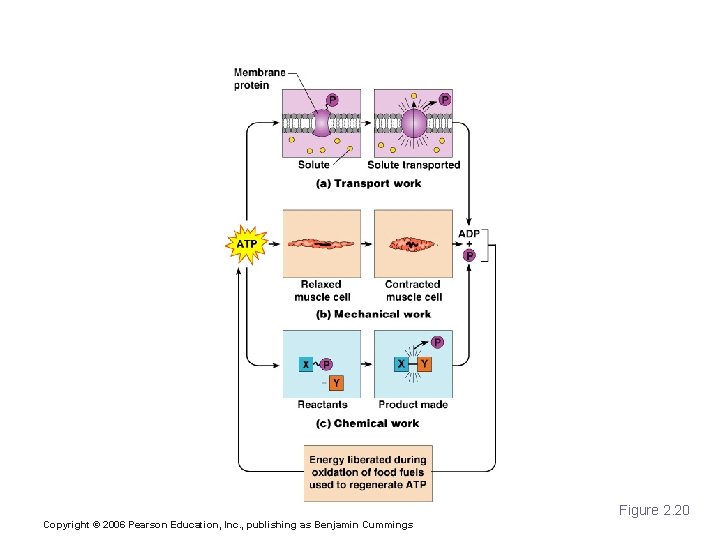
How ATP Drives Cellular Work Figure 2. 20 Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings
 Is anything that occupies space and has mass.
Is anything that occupies space and has mass. Anything that occupies space
Anything that occupies space Anything that occupies space
Anything that occupies space It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter has mass and occupies space
Matter has mass and occupies space Matter anything that takes up space
Matter anything that takes up space Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Block nhĩ thất độ 2 mobitz 1
Block nhĩ thất độ 2 mobitz 1 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Mass
Mass Anything that has mass and take up space
Anything that has mass and take up space Matter is anything that has and takes up
Matter is anything that has and takes up Matter is anything that has
Matter is anything that has Matter is anything that has
Matter is anything that has Matter is anything that?
Matter is anything that? It is anything that has mass and volume
It is anything that has mass and volume Anthing that takes up space and has mass is called?
Anthing that takes up space and has mass is called? Anything that has mass and takes up space
Anything that has mass and takes up space Compare and contrast template
Compare and contrast template Carries energy through matter
Carries energy through matter A disturbance that transmits energy through matter or space
A disturbance that transmits energy through matter or space Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Matter is defined as anything that
Matter is defined as anything that Matter anything that
Matter anything that Is oil more dense than water
Is oil more dense than water Matter is anything that:
Matter is anything that: What is the opposite of sublimation?
What is the opposite of sublimation? No matter anything
No matter anything Matter is anything that
Matter is anything that Matter vs mass
Matter vs mass Matter anything that
Matter anything that Benjamin cummings
Benjamin cummings Matter anything that
Matter anything that Matter anything that
Matter anything that Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Koosha golmohammadi
Koosha golmohammadi Arbor vitae
Arbor vitae Gray matter and white matter
Gray matter and white matter What is gray matter in the brain
What is gray matter in the brain Cartesian space trajectory planning
Cartesian space trajectory planning Space junk the space age began
Space junk the space age began Camera space to world space
Camera space to world space Unscented trajectory chapter 5
Unscented trajectory chapter 5 World space to screen space
World space to screen space 1 pascal a bar
1 pascal a bar Phosphorus cycle
Phosphorus cycle Which reverses the normal flow of thermal energy
Which reverses the normal flow of thermal energy Matter and thermal energy section 1
Matter and thermal energy section 1 Science matter
Science matter Matter energy and measurement
Matter energy and measurement Dark matter and dark energy presentation
Dark matter and dark energy presentation Unit 2 matter and energy
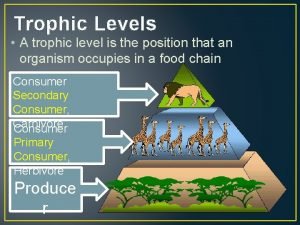
Unit 2 matter and energy What is trophic level
What is trophic level The science duo physical and chemical changes
The science duo physical and chemical changes Solid liquid gas concept map
Solid liquid gas concept map States of matter foldable
States of matter foldable Mechanical wave examples
Mechanical wave examples How do matter and energy interact when waves are generated
How do matter and energy interact when waves are generated Labeled energy pyramid
Labeled energy pyramid Boyle's law k
Boyle's law k A sample of neon gas occupies a volume of 677 ml at 134 kpa
A sample of neon gas occupies a volume of 677 ml at 134 kpa Are gasses highly compressible
Are gasses highly compressible Pv=k boyle's law
Pv=k boyle's law A gas occupies 473 cm3
A gas occupies 473 cm3 A gas occupies 473 cm3 at 36°c. find its volume at 94°c
A gas occupies 473 cm3 at 36°c. find its volume at 94°c A political community that occupies a definite territory
A political community that occupies a definite territory Trophic levels
Trophic levels Within the meninges cerebrospinal fluid occupies the
Within the meninges cerebrospinal fluid occupies the 3 gas laws
3 gas laws Section 1 composition of matter
Section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter