What is MATTER It is anything that occupies















- Slides: 21


What is MATTER? It is anything that occupies space and has mass and weight.

What is MOLECULE? It is formed when two or more atoms join together.

gas ? liquid ? solid ?

What are ATOMS? They are tiny particles and the building blocks of matter. Sub-atomic particles are protons, neutrons, and electrons.

Atoms - Red clay represents the proton - Blue clay represents the neutron - Yellow clay represents the electron

Proton (p) - positively (+) charged - always positioned in the nucleus

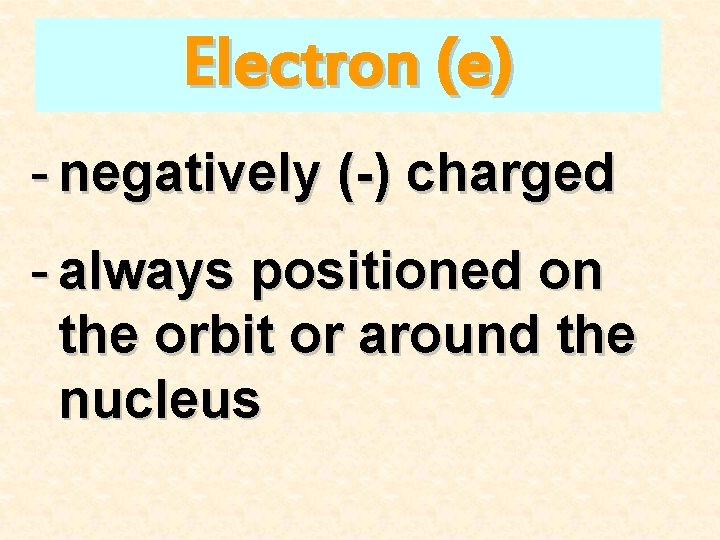
Electron (e) - negatively (-) charged - always positioned on the orbit or around the nucleus

Neutron (n) - has no charge - has the same number of protons and electrons - always positioned in the nucleus, together with the proton


Remember: Properties of atoms are determined by the atomic number. Each element has a corresponding atomic number.

ATOMS

What is an ELEMENT? It is made up of only ONE type of atom therefore, it is a pure substance. Less than 100 different elements occur naturally on Earth, others were made in the laboratories.


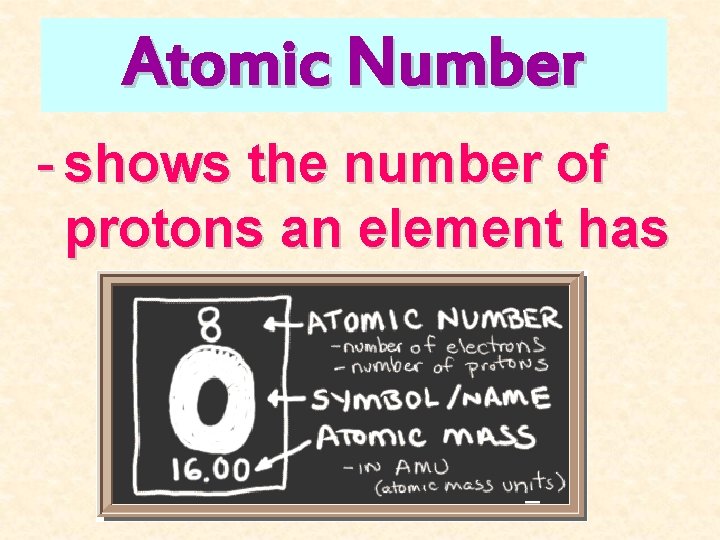
Atomic Number - shows the number of protons an element has

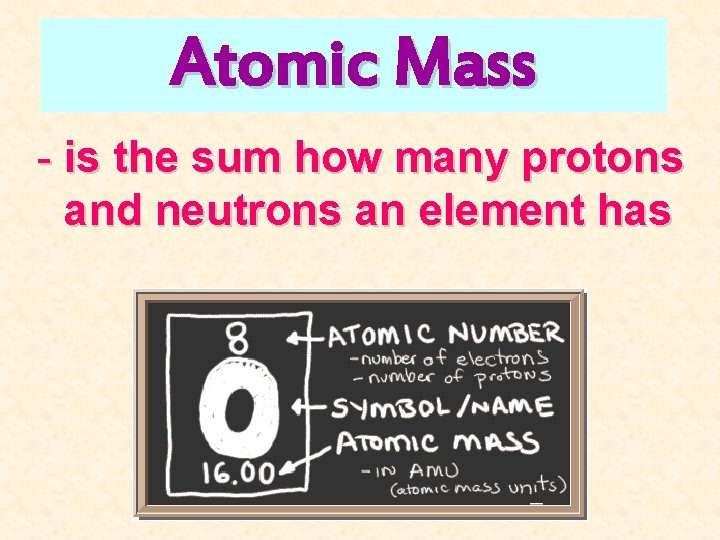
Atomic Mass - is the sum how many protons and neutrons an element has

IF one subatomic particle is removed, would it be the same element? Why not?

What is the importance of knowing the complete subatomic particles in an element?

In life, how can we compare the subatomic particles to you/us?

Seatwork: Draw a if the statement is correct and X if not. 1. The atoms of each element are different from the atoms of all other elements. 2. Elements can be identified by the number of protons found in the nucleus of an atom.

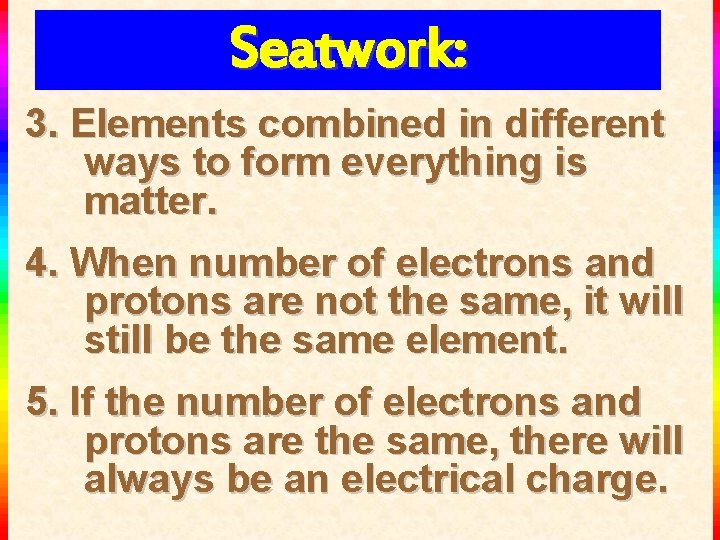
Seatwork: 3. Elements combined in different ways to form everything is matter. 4. When number of electrons and protons are not the same, it will still be the same element. 5. If the number of electrons and protons are the same, there will always be an electrical charge.
 It is anything that has mass and occupies space.
It is anything that has mass and occupies space. Anything that occupies space
Anything that occupies space Matter is anything that occupies space and has -----------
Matter is anything that occupies space and has ----------- It is anything that has mass and occupies space
It is anything that has mass and occupies space It is anything that has mass and occupies space
It is anything that has mass and occupies space Tìm vết của đường thẳng
Tìm vết của đường thẳng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Tôn thất thuyết là ai
Tôn thất thuyết là ai Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Walmart thất bại ở nhật
Walmart thất bại ở nhật Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block xoang nhĩ
Block xoang nhĩ What is the opposite of sublimation?
What is the opposite of sublimation? Matter anything that
Matter anything that Something that takes up space
Something that takes up space Matter anything that
Matter anything that Matter is anything that...
Matter is anything that... Is anything that has mass and takes up space.
Is anything that has mass and takes up space. No matter anything
No matter anything