Matter The Nature of Matter is anything that

















- Slides: 24

Matter

The Nature of Matter is anything that takes up space and has mass. The word matter comes from the Latin word materia, meaning “material” or “stuff” First we need Examples of Matter : to ask. . . What is matter? You can observe matter easily with your senses. . . rocks, trees, bicycles, air. . . Basically everything and anything! The only thing that wouldn’t be matter would be energy (sunlight, heat, electricity). - no mass or volume so they can’t be matter!

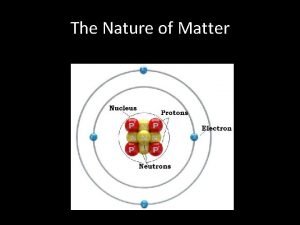
Elements, Molecules, and Compounds Elements and compounds make up all the different kinds of matter in the universe. Elements are the simplest form of matter Cannot be broken down into simpler substances. Six elements make up: 99% of all living matter! Examples of Elements n Each element is made of atoms of the same type. • Sulfur • Nitrogen ün Each gold has a unique ü aluminum set of physical and chemical • Potassium • Carbon properties. ü silver ü nitrogen • n. Oxygen • Hydrogen 117 known elements in the universe. ü oxygen ü tin n Approximately 92 are found naturally on Earth. ü hydrogen ü calcium n

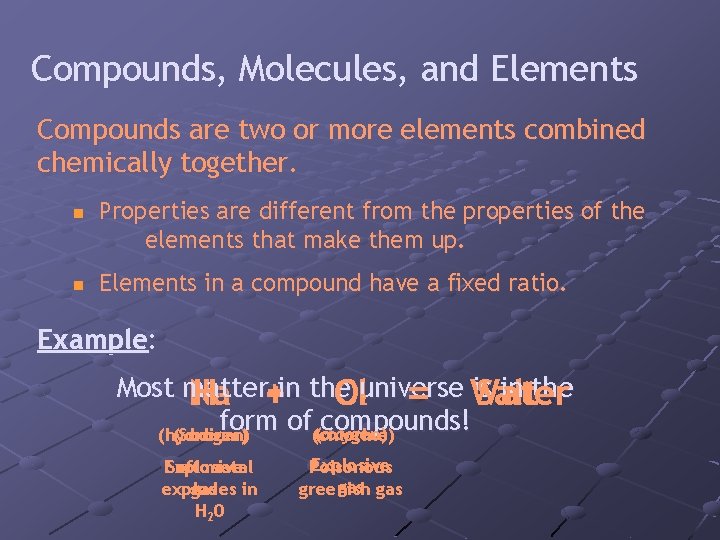
Compounds, Molecules, and Elements Compounds are two or more elements combined chemically together. n n Properties are different from the properties of the elements that make them up. Elements in a compound have a fixed ratio. Example: Most matter is in the Na H + O Cluniverse 2 = Water Salt +in the form of(chlorine) compounds! (oxygen) (hydrogen) (Sodium) Explosive Soft metal explodes gas in H 2 0 Explosive Poisonous gas greenish

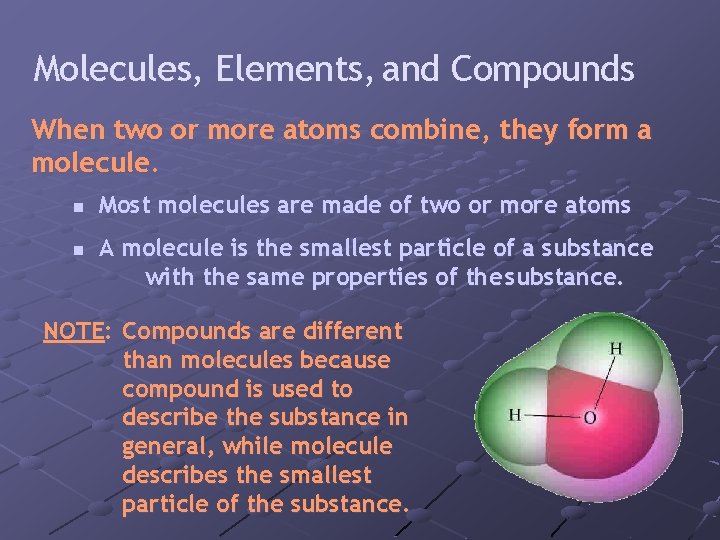
Molecules, Elements, and Compounds When two or more atoms combine, they form a molecule. n n Most molecules are made of two or more atoms A molecule is the smallest particle of a substance with the same properties of the substance. NOTE: Compounds are different than molecules because compound is used to describe the substance in general, while molecule describes the smallest particle of the substance.

Mixtures, Solutions, and Suspensions A mixture is a combination of two or more substances NOT combined chemically. May be a mixture of both elements and compounds n Substances keep their unique properties and can be separated by physical means. n

Mixtures, Solutions, and Suspensions There are two types of mixtures. . . Heterogeneous – the parts of the mixture are noticeably different from one another. Homogeneous – the parts (substances) are evenly distributed. It is difficult to tell one substance from another.

Solutions, and Suspensions and Colloids A solution is a mixture that looks like a single substance and has the same properties throughout. Solute ~ The substance that dissolves in a solution. Solvent ~ The substance into which the solute dissolves.

Solutions, and Suspensions and Colloids A colloid is a mixture that contains both small particles in solution and larger particles in suspension. • Colloids do not separate into layers. • Colloids, like suspensions scatter light. Milk is an example of a colloid. In a suspension components are dispersed, but large enough to see and settle out.

Properties of Matter

Physical Properties Physical property is a property that can be observed without changing the identity of the substance. Examples: ü viscosity ü conductivity ü malleability ü hardness ü magnetism ü melting point ü boiling point ü density ü color

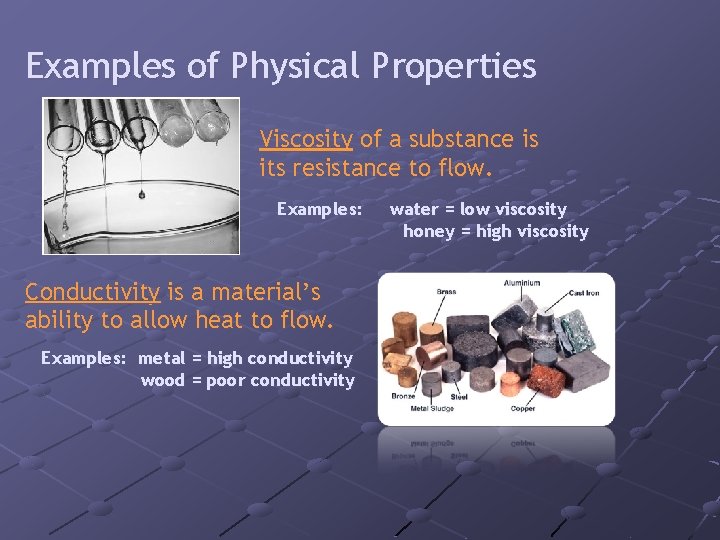
Examples of Physical Properties Viscosity of a substance is its resistance to flow. Examples: Conductivity is a material’s ability to allow heat to flow. Examples: metal = high conductivity wood = poor conductivity water = low viscosity honey = high viscosity

Examples of Physical Properties Malleability of a substance is its ability to be hammered into a thin sheet Melting and Boiling points are the temperatures at which a solid becomes a liquid and a liquid becomes a gas. Density of a substance is the ratio of its mass compared to its volume.

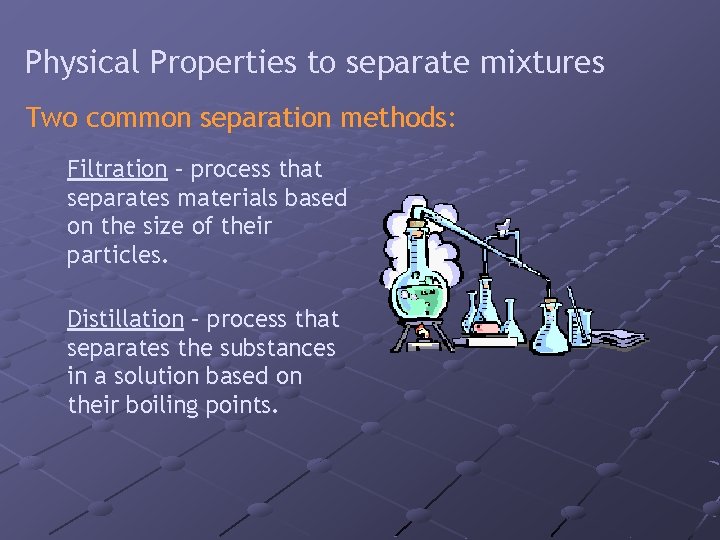
Physical Properties to separate mixtures Two common separation methods: Filtration – process that separates materials based on the size of their particles. Distillation – process that separates the substances in a solution based on their boiling points.

Physical Change A change in the appearance, without changing the composition of the material. be reversible , or irreversible It is • a. Can physical change if. . . • Substance may seem different, but the atoms link upor is size the same. changes shape It ü way Ordissolves. the substance changes phase.

Chemical Properties

Chemical Properties Chemical property is any ability to produce a change in the composition of matter. Examples of chemical properties. . . flammability reactivity Material’s ability to burn in the presence of oxygen. How readily a substance combines chemically with other substances.

Chemical Changes Chemical changes occur when a substance reacts and forms one or more new substances. You know a chemical change has occurred when. . . ü A change in color. ü Production of a gas. ü Formation of a precipitate.

What kind of change is it? physical

What kind of change is it? chemical

What kind of change is it? physical

What kind of change is it? physical

What kind of change is it? chemical

What kind of change is it? physical
 Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Tôn thất thuyết là ai
Tôn thất thuyết là ai Phân độ lown
Phân độ lown Walmart thất bại ở nhật
Walmart thất bại ở nhật Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block nhĩ thất độ 2 mobitz 2
Block nhĩ thất độ 2 mobitz 2 Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Matter vs mass
Matter vs mass Benjamin cummings
Benjamin cummings Use of density
Use of density What has mass and occupies space
What has mass and occupies space Matter is anything that
Matter is anything that Matter is anything that occupies space and has mass
Matter is anything that occupies space and has mass Matter anything that
Matter anything that Masses defintion
Masses defintion Matter is anything that has
Matter is anything that has Defintion of matter
Defintion of matter Matter is anything that has
Matter is anything that has Matter is defined as anything that
Matter is defined as anything that No matter anything
No matter anything Matter is anything that
Matter is anything that Anything that has mass and takes up space
Anything that has mass and takes up space