What is matter Matter is anything that takes




























- Slides: 65

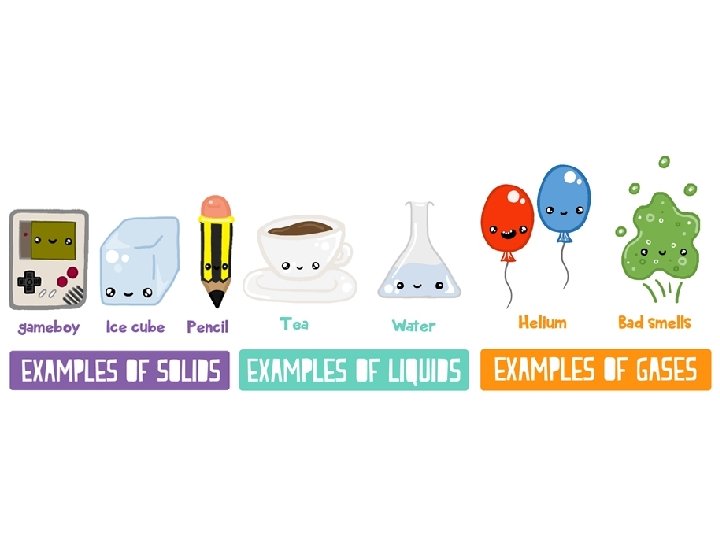
What is matter? • Matter is anything that takes up space and has mass. • Write some examples of matter in this room… • Matter can have many different properties (characteristics that can be used to identify and classify it)




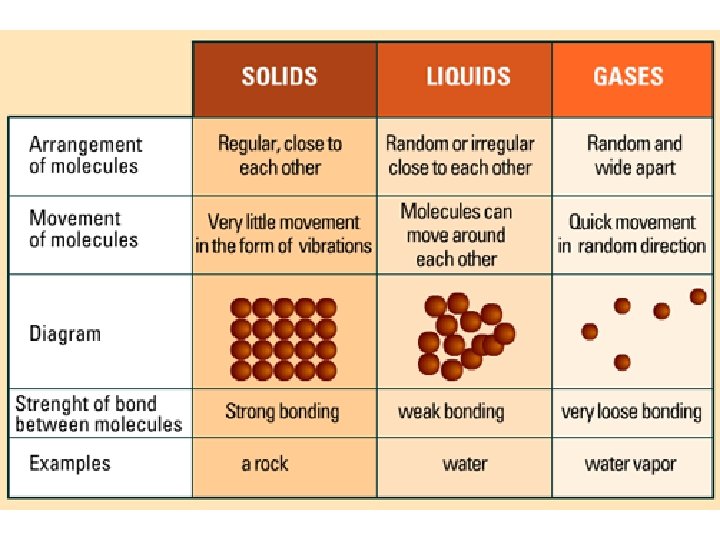
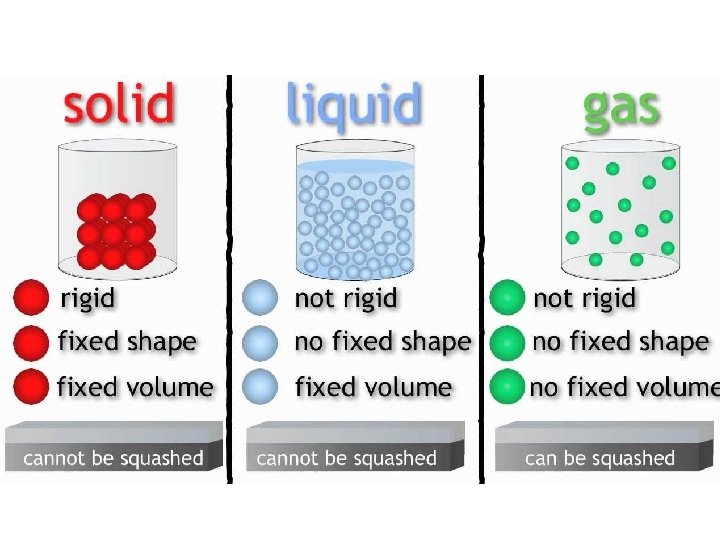
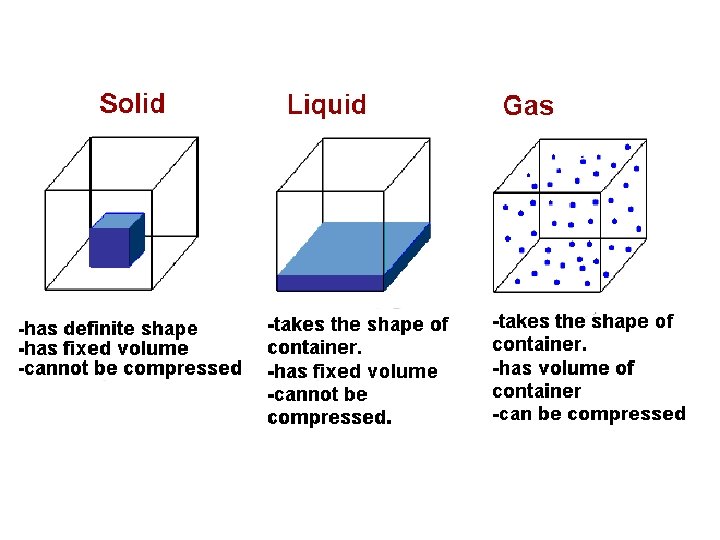
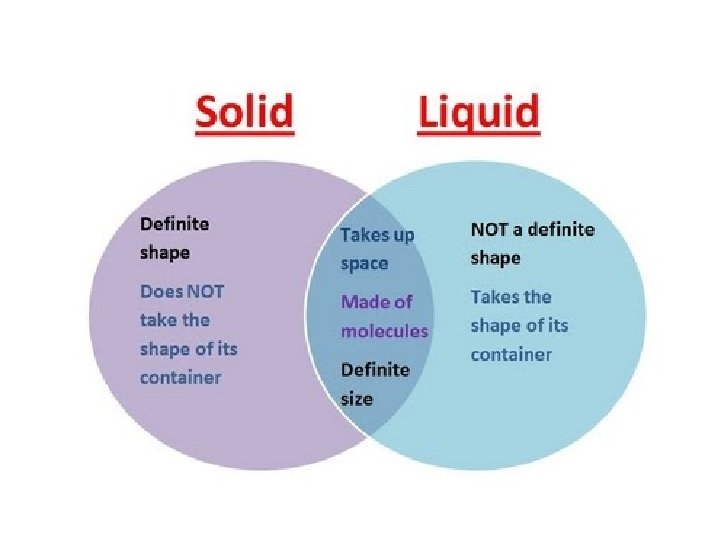
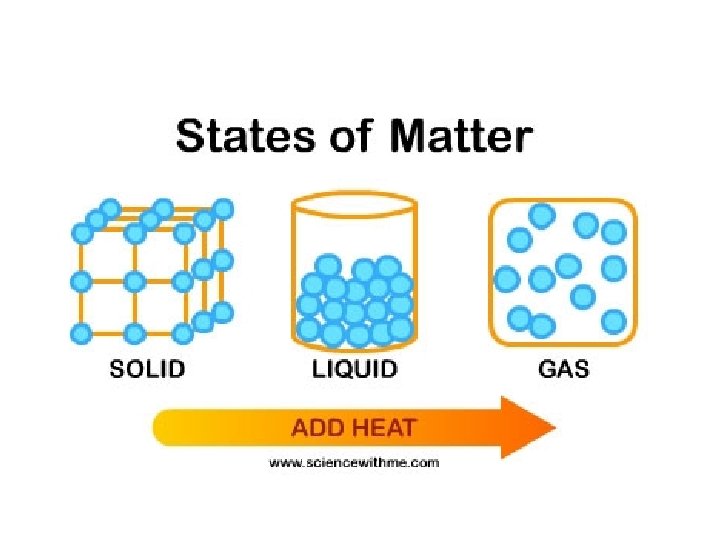
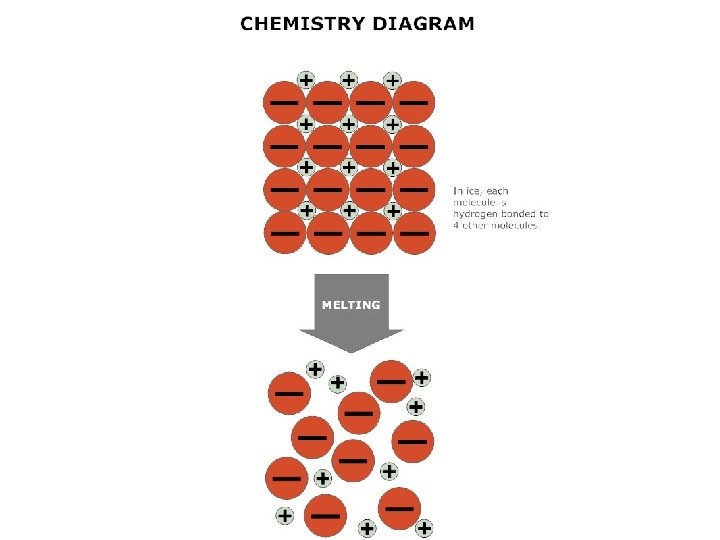
solids shape • A solid has a definite _______ and _______ volume – (“Definite” means you can tell what it is & it is the same) • A solid keeps its shape and volume no matter what it’s in • The particles are closely packed together • The particles do move some: they vibrate in place, but do not move other than that


liquids volume • A liquid has a definite _______ but not a definite ______ shape – The shape of a liquid changes with its container • Particles in a liquid still touch but move freely past each other • Liquids are fluids (a fluid is something that flows)


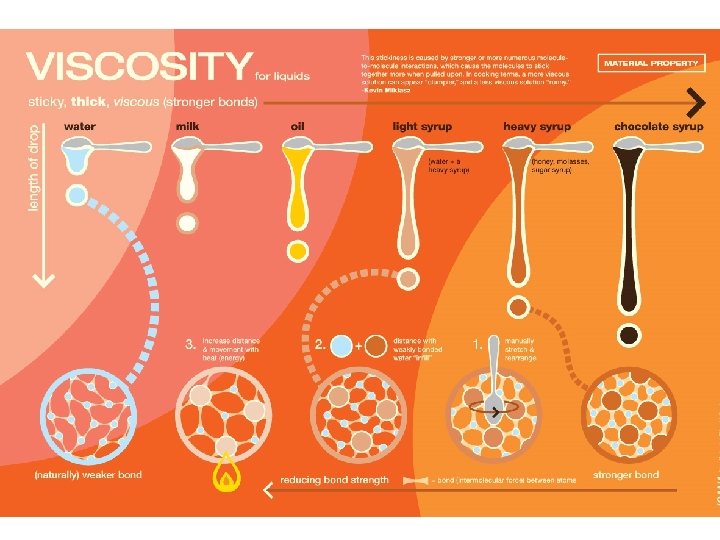
Properties of liquids • Water molecules are strongly attracted to each other • Surface tension: an inward force that pulls liquid molecules together, forming puddles or drops – Surface tension makes the surface of water act kind of like a skin, so things can float on (and even walk on) water • Viscosity is a liquid’s resistance to flowing (how slowly it flows) – Viscosity depends on the size and shape of the liquid’s particles – Different liquids have different viscosities • Examples: water flows differently than honey because they have different viscosities




Non-newtonian Fluids • ketchup • Ants • Mytbusters walk on water • Oobleck on a speaker in slo mo

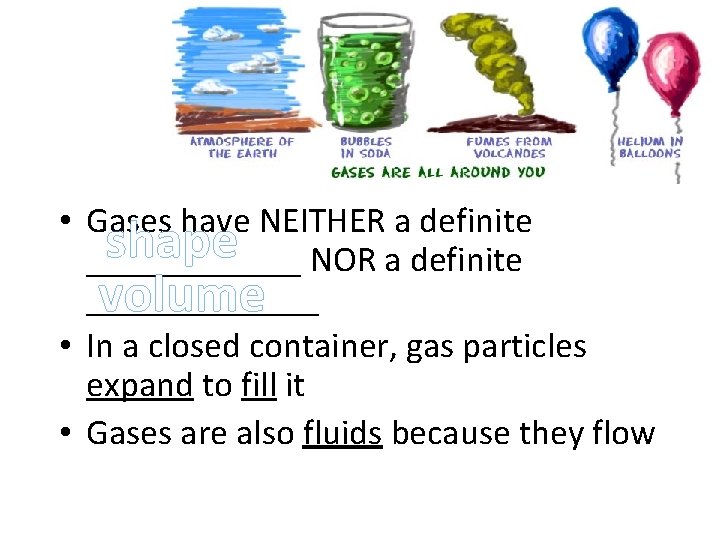
gases • Gases have NEITHER a definite shape NOR a definite _____________ volume • In a closed container, gas particles expand to fill it • Gases are also fluids because they flow

Properties of gases • Volume: amount of SPACE matter takes up • The Volume of a gas is the same as the volume of its container • Most of the volume of a gas is empty space • Gases can be compressed (squished) to fill smaller volumes (compressed air is used to fill balloons) – When a gas is compressed, the particles do not change size, they just move farther away from each other

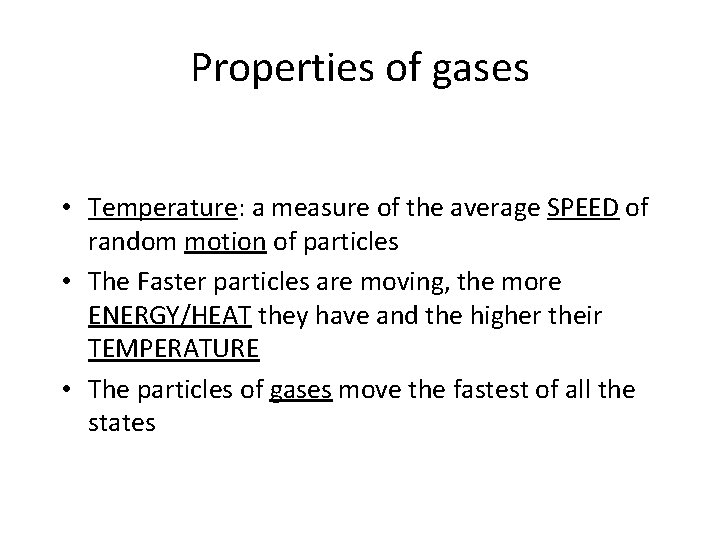
Properties of gases • Temperature: a measure of the average SPEED of random motion of particles • The Faster particles are moving, the more ENERGY/HEAT they have and the higher their TEMPERATURE • The particles of gases move the fastest of all the states


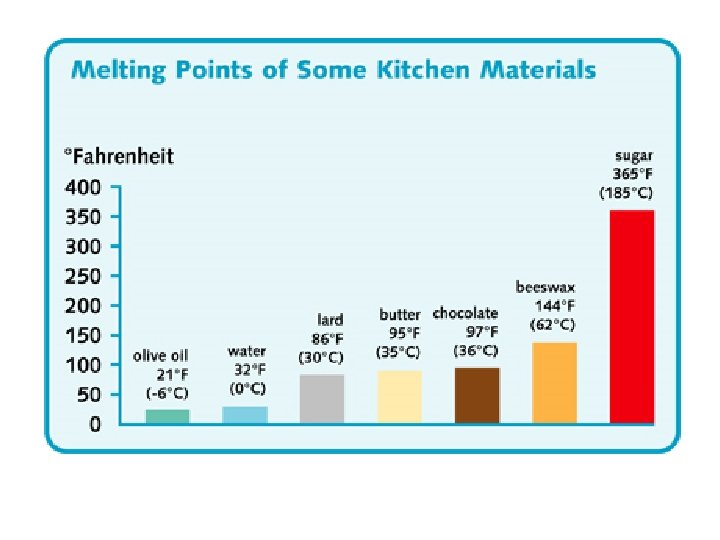
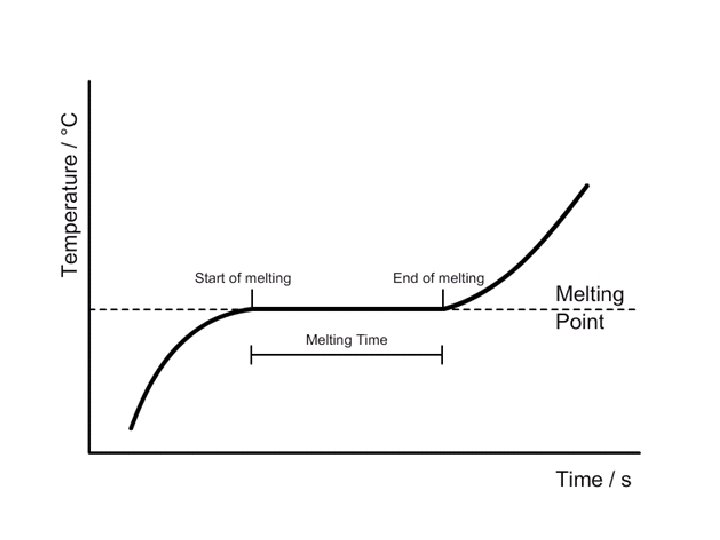
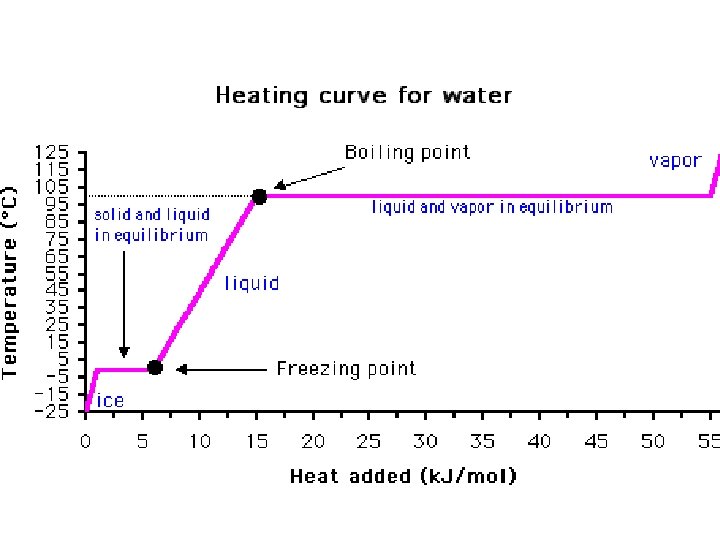
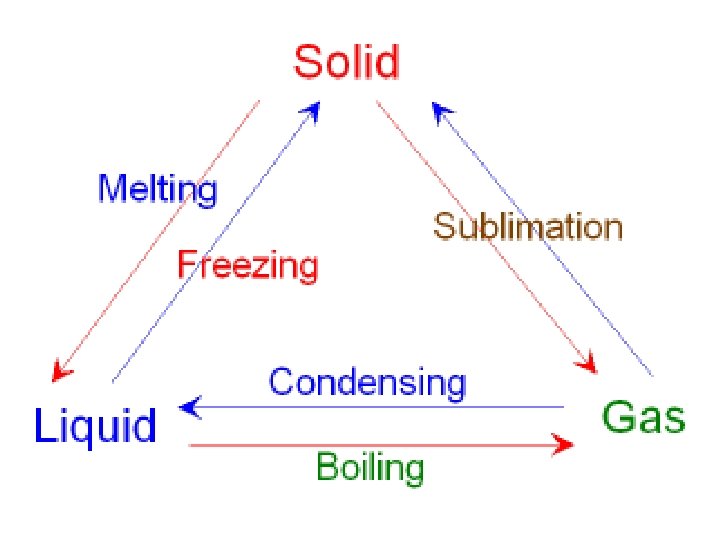
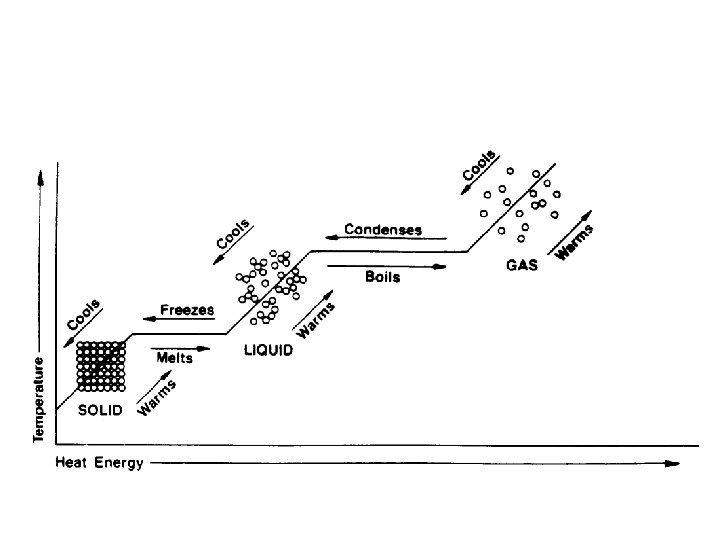
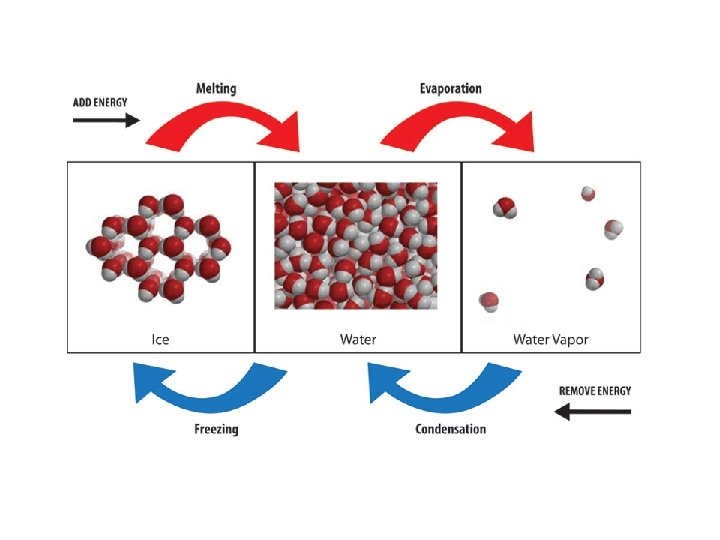
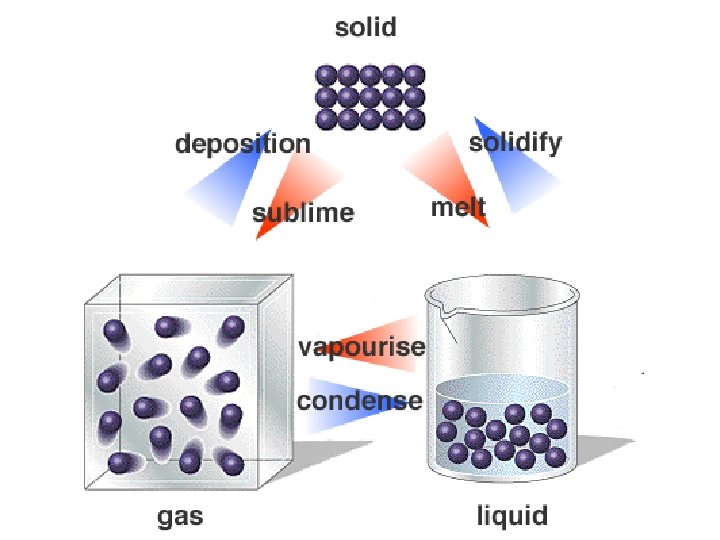
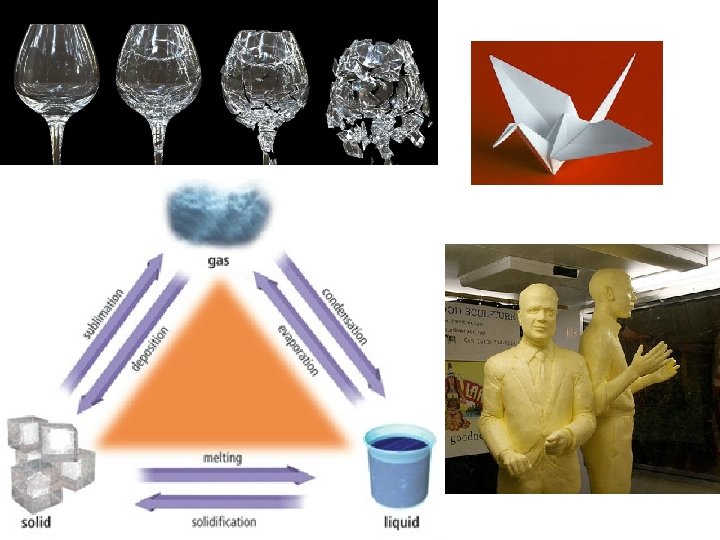
melting • Changing state from solid liquid • Pure, crystalline solids melt at specific temperatures called melting points • This is a physical property • The melting point of water is 0 degrees Celsius (32 deg. F) – It stays at 0 degrees until it is done freezing • As a solid melts, its particles begin to move more, and they break away from their fixed positions and can move more freely




freezing • Liquid solid • Reverse of melting • At the freezing point, particles move so slowly they start to get stuck in a fixed position – Particles lose energy • Some things become solid (freeze) at relatively high temps – Ex: chocolate, candle wax, butter

vaporization • Changing state from liquid to gas is called vaporization • This happens when the particles in the liquid gain enough energy to move independently (become a gas) • 2 types of vaporization: evaporation & boiling

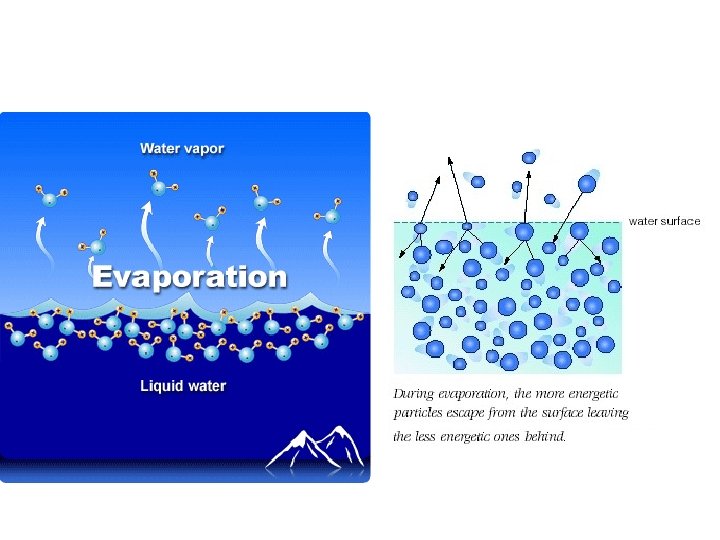
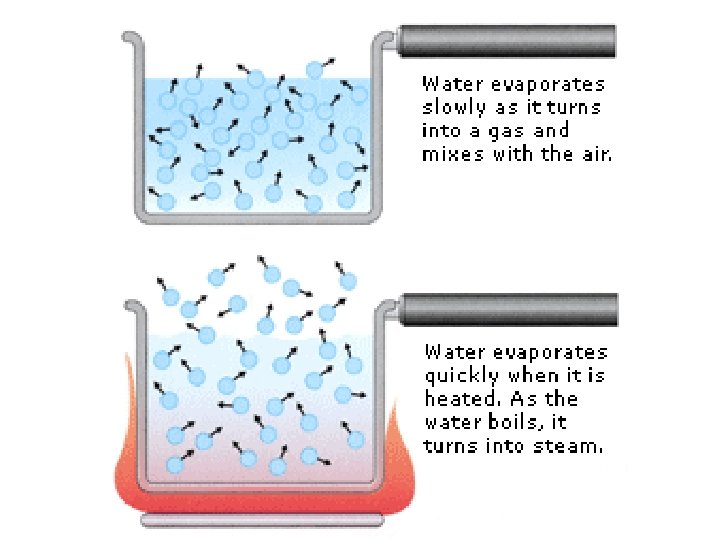
evaporation • Evaporation is vaporization that takes place on the surface of a liquid • Can take place at any temperature • Ex: shrinking puddle


boiling • Boiling is vaporization that takes place all over the liquid • Forms bubbles below the surface that rise and escape • Temperature at which a liquid boils is its boiling point • Happens faster than evaporation



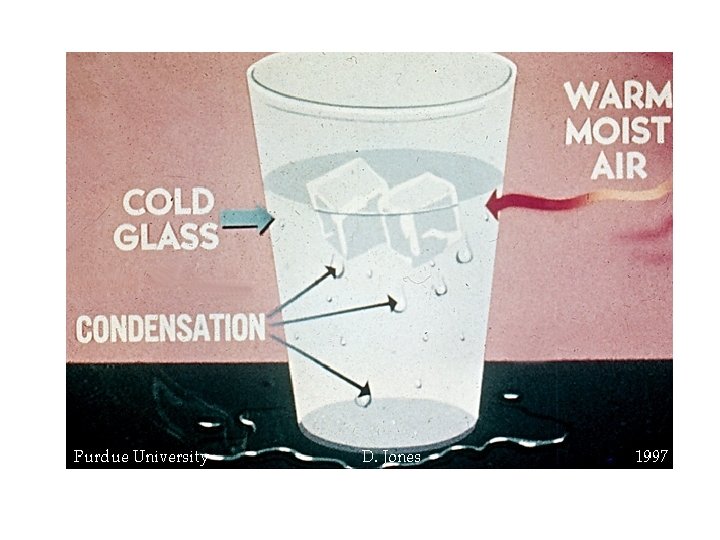
condensation • Changing state from a gas liquid • Opposite of vaporization • Occurs when particles of a gas lose enough energy to become liquid • Ex: breathing on a mirror, glasses fogging up, clouds forming (water vapor condensing into liquid droplets—true water vapor is invisible)


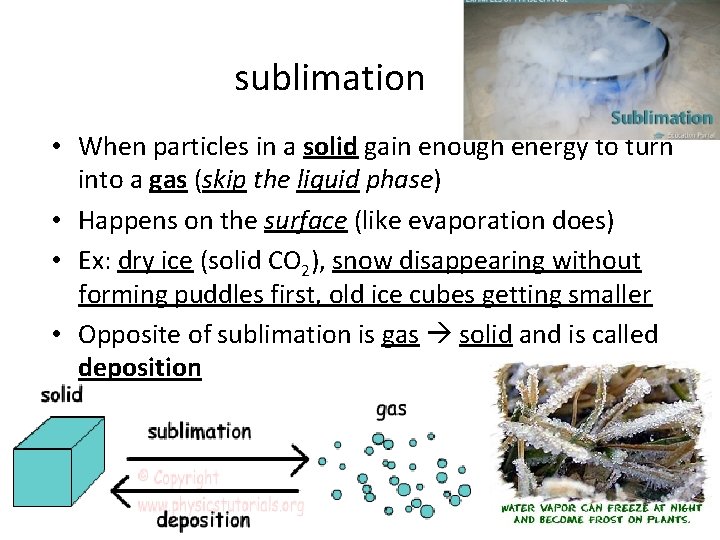
sublimation • When particles in a solid gain enough energy to turn into a gas (skip the liquid phase) • Happens on the surface (like evaporation does) • Ex: dry ice (solid CO 2), snow disappearing without forming puddles first, old ice cubes getting smaller • Opposite of sublimation is gas solid and is called deposition





Properties of matter • Used to identify and classify matter. • What are some examples of properties of matter? – Temperature, color, texture, phase (solid/liquid/gas), flammability, etc. • Chemistry is the study of matter and how it changes. • A substance is made of pure matter. It is a single kind of matter and always has the same composition (makeup). – Example: table salt, pure water

Physical Properties of matter • Matter has physical properties and chemical properties. • A physical property is a characteristic that can be observed without changing the substance into another substance. – Examples? – Luster (shininess), hardness, texture, color, temperature, conductivity (of heat or electricity), solubility, flexibility (malleability or ductility), freezing point, boiling point, melting point, size (area/length/mass/volume/etc. ), brittleness, concentration, density, electrical charge, location, luminance, momentum, opacity, specific heat, velocity, etc. .


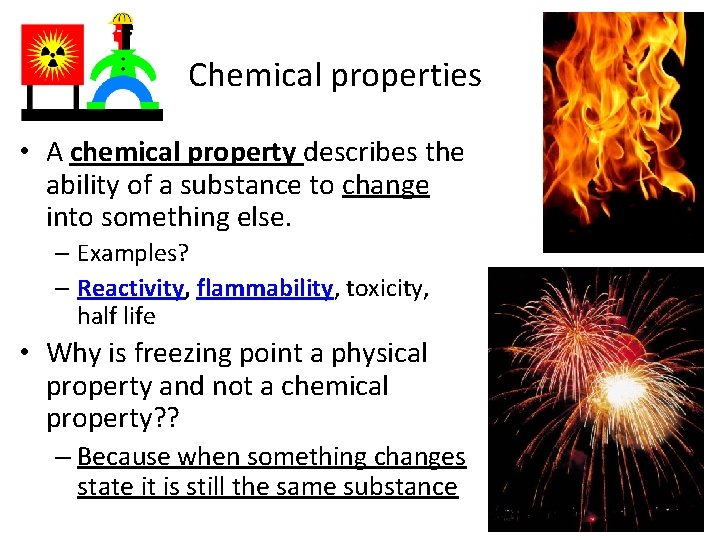
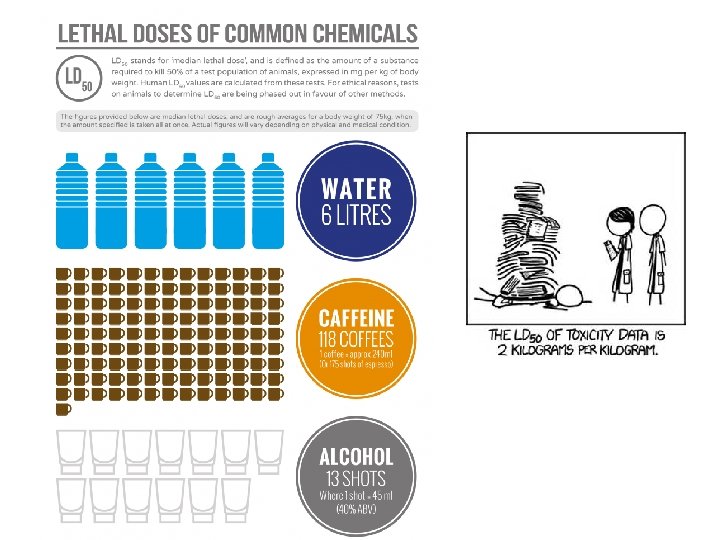
Chemical properties • A chemical property describes the ability of a substance to change into something else. – Examples? – Reactivity, flammability, toxicity, half life • Why is freezing point a physical property and not a chemical property? ? – Because when something changes state it is still the same substance


Changes in Matter

Physical Changes • A physical changes the form (shape) or appearance of matter but NOT what it is made of – It is still the same substance – Physical changes change physical properties and do NOT change chemical properties • Examples of physical changes? – Crushing, dissolving, bending, tearing – Any changes of state/phase (boiling, melting, evaporating, etc. )


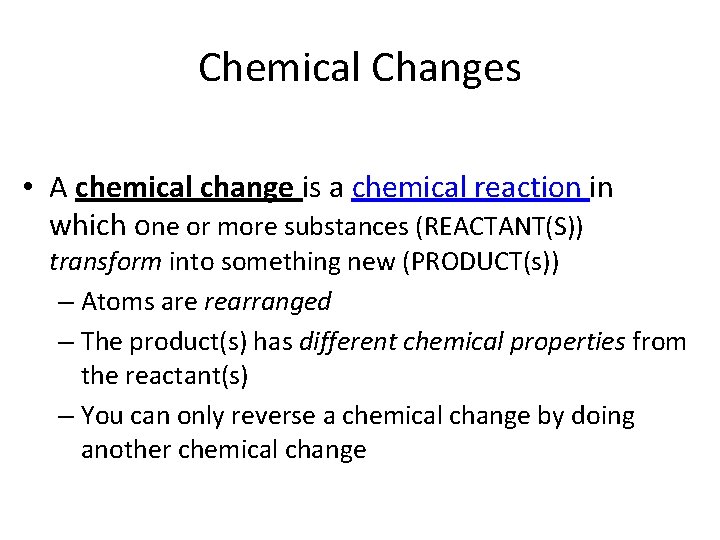
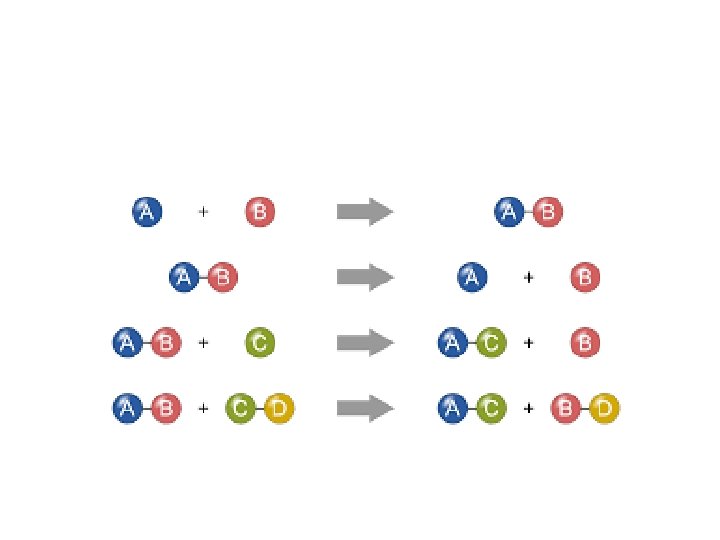
Chemical Changes • A chemical change is a chemical reaction in which one or more substances (REACTANT(S)) transform into something new (PRODUCT(s)) – Atoms are rearranged – The product(s) has different chemical properties from the reactant(s) – You can only reverse a chemical change by doing another chemical change


Chemical Changes • Examples of chemical changes? – Photosynthesis, combustion, decomposition, fermentation, rusting/oxidation, cooking, tarnishing, and any other chemical reaction


Physical or Chemical Change?


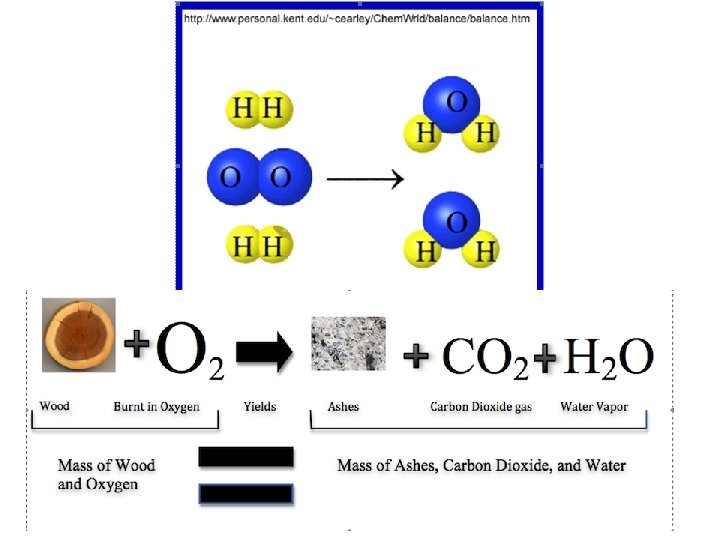
Law of Conservation of Mass • In a chemical change, the mass of the products ALWAYS equals the mass of the reactants – This is called the Law of Conservation of Mass • Matter is NEVER created or destroyed— atoms are only rearranged

Video

Endothermic Changes • (Or endothermic reactions) • Endo = inside • A chemical change in which energy is absorbed – Makes surroundings cooler • Examples? – Instant ice pack, ice melting (ice + energy = water)

Exothermic Changes • Or exothermic reactions • Exo = outside • A chemical change that releases energy – Makes surroundings warmer • Examples? – Combustion – Instant hand warmer

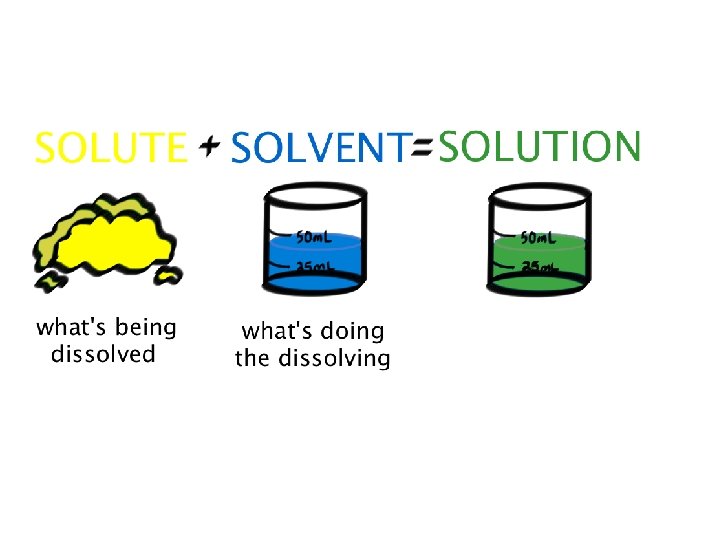
Solubility • Solubility is a physical property that describes how well a substance can dissolve in something else • The substance being dissolved is the solute • The substance doing the dissolving (that the solute is dissolved in) is the solvent • Water is a “universal” solvent because most things will dissolve in it



Factors affecting Solubility 1. Temperature of solvent – Solutes usually dissolve better in hot solvents – Ex: hot cocoa; ice vs. hot tea

Factors affecting Solubility 2. Surface area of solute – Powders usually dissolve better than large chunks – Ex: sugar cubes vs. granulated sugar

Factors affecting solubility 3. Stirring –Stirring helps a solute dissolve faster

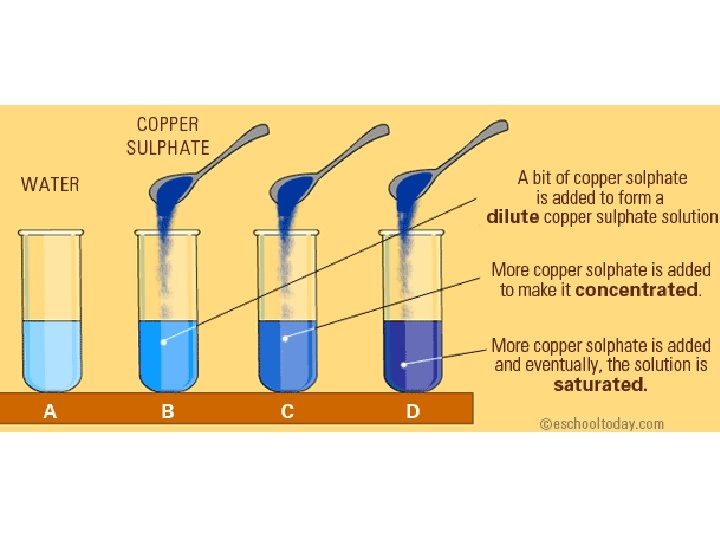
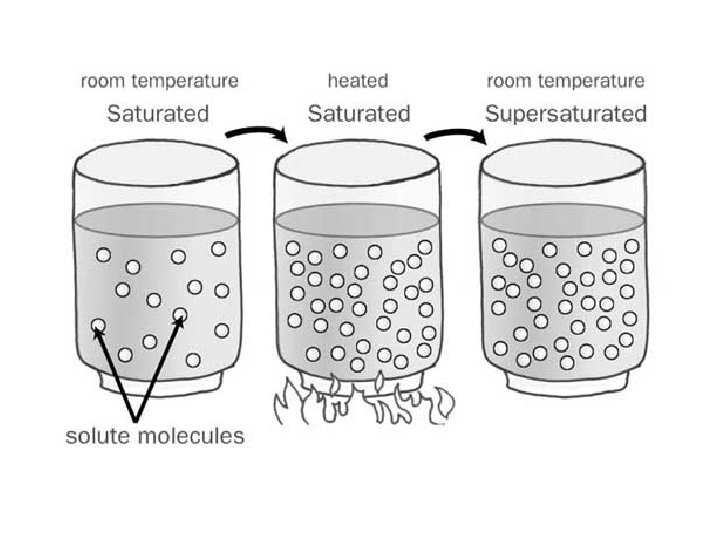
Types of solutions • Unsaturated: more solute can still be dissolved • Saturated: the solvent has as much solute as it can hold • Supersaturated: the solvent contains more solute than its capacity



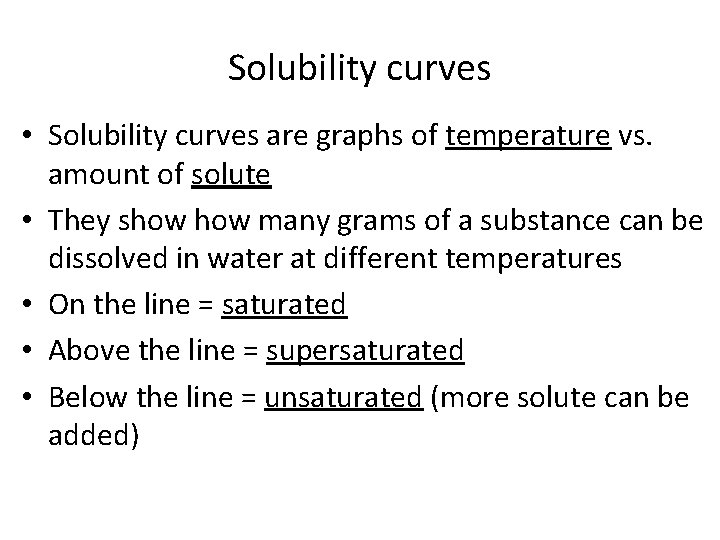
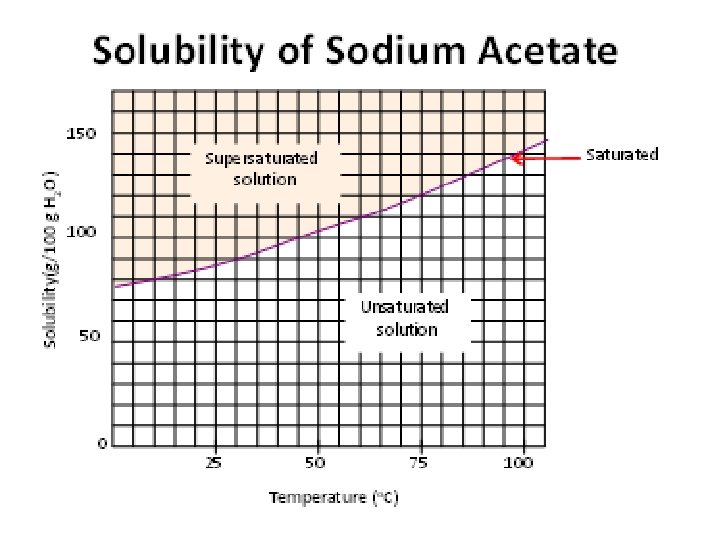
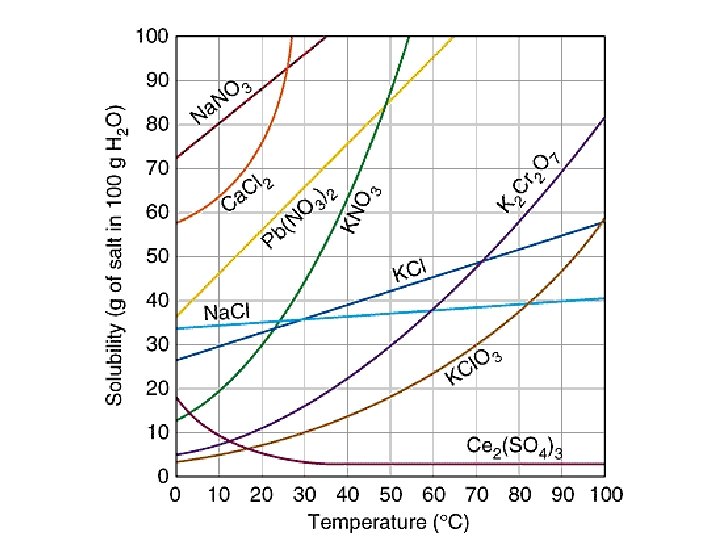
Solubility curves • Solubility curves are graphs of temperature vs. amount of solute • They show many grams of a substance can be dissolved in water at different temperatures • On the line = saturated • Above the line = supersaturated • Below the line = unsaturated (more solute can be added)


 Anything that occupies space and has mass is called
Anything that occupies space and has mass is called Defintion of malleability
Defintion of malleability Anything that has mass and takes up space
Anything that has mass and takes up space Matter anything that takes up space
Matter anything that takes up space Anything that has mass and takes up space
Anything that has mass and takes up space Anything that has mass and volume is
Anything that has mass and volume is Anything that has mass and takes up space
Anything that has mass and takes up space Anthing that takes up space and has mass is called?
Anthing that takes up space and has mass is called? Matter is anything that has both
Matter is anything that has both Anything that has mass and takes up space
Anything that has mass and takes up space Anything that has mass and volume
Anything that has mass and volume Block nhĩ thất độ 2 mobitz 2
Block nhĩ thất độ 2 mobitz 2 Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Tôn thất thuyết là ai
Tôn thất thuyết là ai Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Phân độ lown
Phân độ lown Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Matter anything that
Matter anything that Matter anything that
Matter anything that Dmv formula
Dmv formula Matter is anything that
Matter is anything that Matter is defined as anything that
Matter is defined as anything that Matter anything that
Matter anything that Is oil more dense than water
Is oil more dense than water Matter anything that
Matter anything that No matter anything
No matter anything Matter is anything that has
Matter is anything that has Matter is anything that
Matter is anything that Benjamin cummings
Benjamin cummings Matter is anything that occupies space and has -----------
Matter is anything that occupies space and has ----------- Matter is anything that
Matter is anything that Matter is anything that:
Matter is anything that: Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Matter
Matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter What makes up the diencephalon
What makes up the diencephalon Flow of energy vs flow of matter
Flow of energy vs flow of matter White matter
White matter The characters struggle takes place
The characters struggle takes place Battling over clayoquot big trees
Battling over clayoquot big trees It takes different strokes to rule the world
It takes different strokes to rule the world Ratatouille worksheet answers
Ratatouille worksheet answers Chichi takes on nnn
Chichi takes on nnn The roman world takes shape
The roman world takes shape Internal sorting takes place in
Internal sorting takes place in It takes two
It takes two Communication takes place between
Communication takes place between Everyone has what it takes
Everyone has what it takes A person or animal who takes part in a story
A person or animal who takes part in a story Random orientation of homologous chromosomes
Random orientation of homologous chromosomes They say sometimes you win some
They say sometimes you win some Take away sins
Take away sins Tucker turtle takes time to tuck and think video
Tucker turtle takes time to tuck and think video The lamb of god who takes away the sins of the world
The lamb of god who takes away the sins of the world Conflict takes place outside of the body.
Conflict takes place outside of the body. Define electro static force
Define electro static force Dr chus
Dr chus Singular verb
Singular verb You don't play the piano question tag
You don't play the piano question tag