MATTER MATTER ANYTHING MASS THAT TAKES UP SPACE











- Slides: 23

MATTER

MATTER � ANYTHING MASS THAT TAKES UP SPACE AND HAS STATE OF MATTER IS DETERMINED BY: THE MOTION OF THE PARTICLES AND THE STRENGTH OF ATTRACTION BETWEEN PARTICLES

ALL MATTER IS MADE OF PARTICLES �ATOMS �MOLECULES �IONS

SOLIDS � MATTER WITH A DEFINITE SHAPE � PARTICLES ARE PACKED CLOSELY TOGETHER AND VIBRATE IN PLACE.

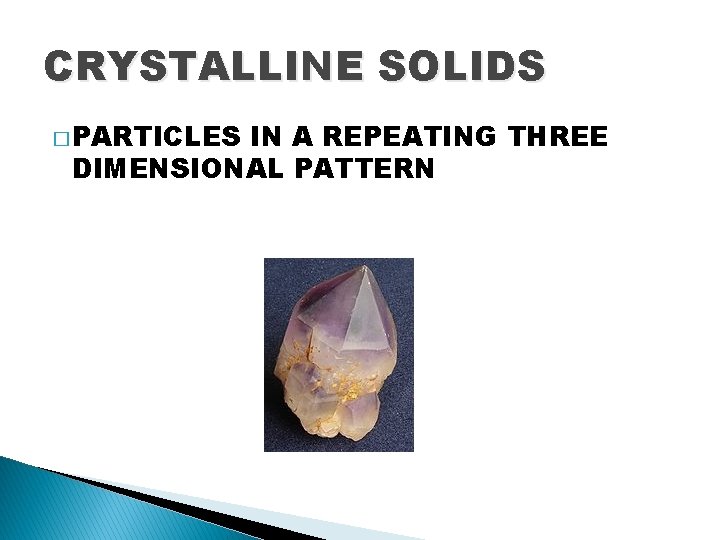
CRYSTALLINE SOLIDS � PARTICLES IN A REPEATING THREE DIMENSIONAL PATTERN

AMORPHOUS SOLIDS � PARTICLES ARE IN A RANDOM ARRANGEMENT � EXAMPLES: GLASS, RUBBER, PLASTIC

LIQUIDS � THEY HAVE A DEFINITE VOLUME BUT NO DEFINITE SHAPE. � THE PARTICLES MOVE ABOUT MORE FREELY THAN SOLIDS.

VISCOSITY �A LIQUIDS RESISTANCE TO FLOW. � THE SLOWER A LIQUID FLOWS THE HIGHER ITS VISCOSITY. � VISCOSITY COLDER. INCREASES AS THE LIQUID GETS

SURFACE TENSION � PARTICLES ON THE SURFACE ARE PULLED TOGETHER TOWARD THE CENTER.

GASES � PARTICLES DO NOT HAVE A DEFINITE SHAPE OR VOLUME. � THE PARTICLES MOVE OUT FARTHER THAN LIQUIDS AND SOLIDS. THEY MOVE AT HIGH SPEEDS IN ALL DIRECTIONS.

PLASMA � SIMILAR � THE TO A GAS ATOMS OR MOLECULES HAVE IONIZED (THE NUMBER OF ELECTRONS HAS BEEN REDUCED OR INCREASED)

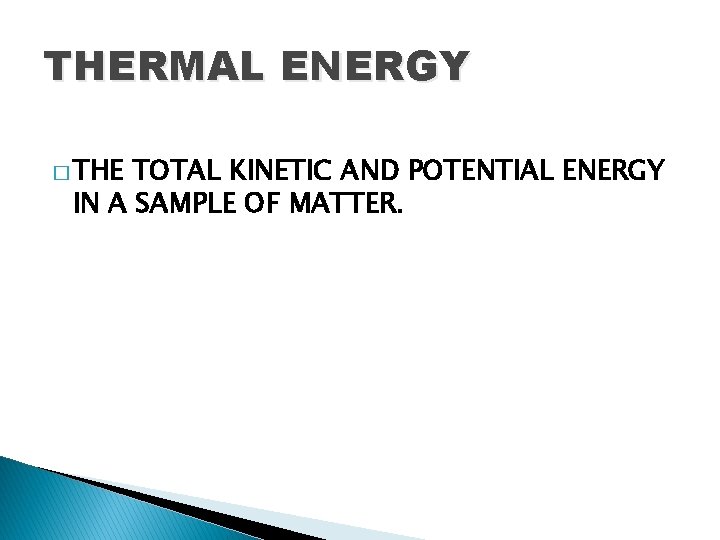
THERMAL ENERGY � THE TOTAL KINETIC AND POTENTIAL ENERGY IN A SAMPLE OF MATTER.

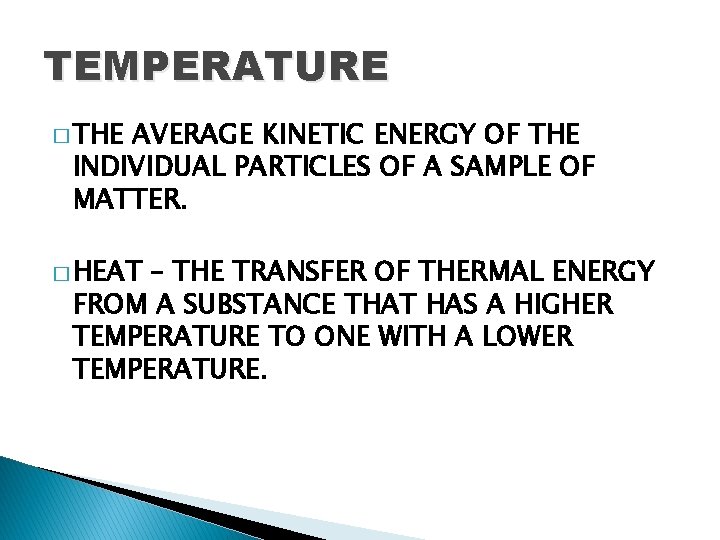
TEMPERATURE � THE AVERAGE KINETIC ENERGY OF THE INDIVIDUAL PARTICLES OF A SAMPLE OF MATTER. � HEAT – THE TRANSFER OF THERMAL ENERGY FROM A SUBSTANCE THAT HAS A HIGHER TEMPERATURE TO ONE WITH A LOWER TEMPERATURE.

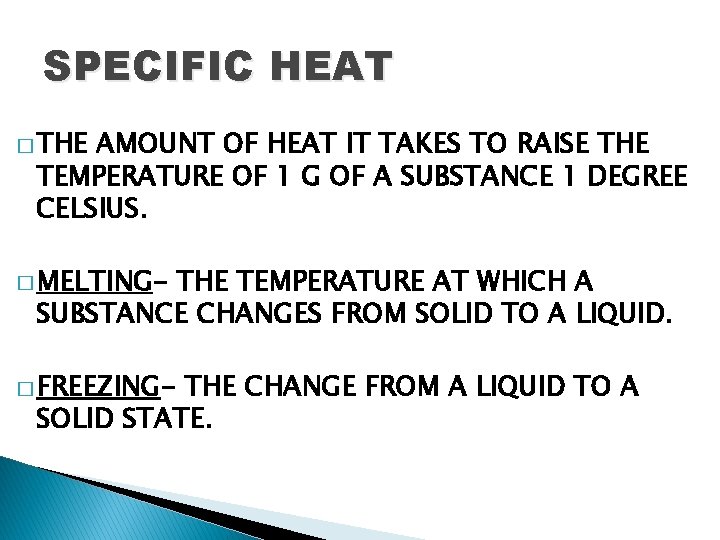
SPECIFIC HEAT � THE AMOUNT OF HEAT IT TAKES TO RAISE THE TEMPERATURE OF 1 G OF A SUBSTANCE 1 DEGREE CELSIUS. � MELTING- THE TEMPERATURE AT WHICH A SUBSTANCE CHANGES FROM SOLID TO A LIQUID. � FREEZING- THE CHANGE FROM A LIQUID TO A SOLID STATE.

VAPORIZATION � THE CHANGE OF A SUBSTANCE FROM A LIQUID TO A GAS STATE. � BOILING POINT – THE TEMPERATURE AT WHICH A LIQUID BOILS. � EVAPORATION- TAKES PLACE AT THE SURFACE OF A LIQUID AND CAN TAKE PLACE BELOW THE BOILING POINT.

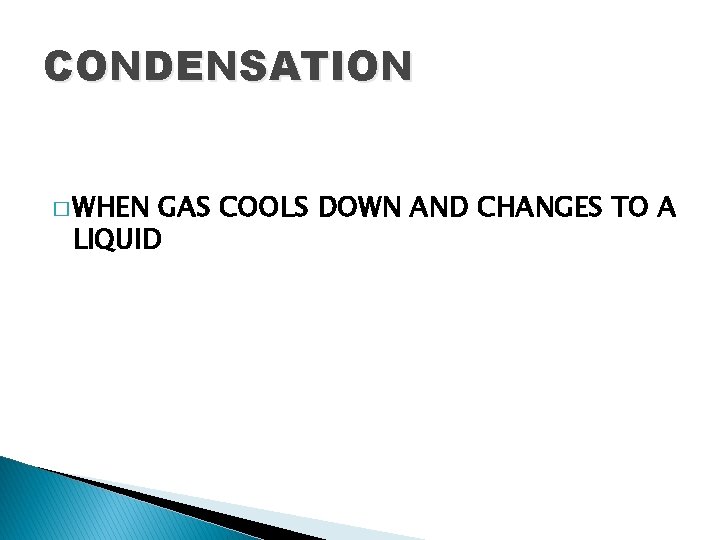
CONDENSATION � WHEN GAS COOLS DOWN AND CHANGES TO A LIQUID

NEWTONS �UNIT OF MEASURE FORCE

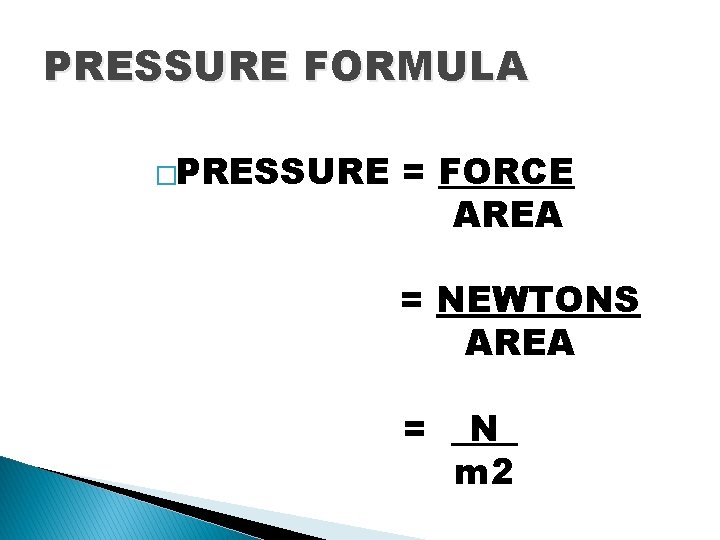
PRESSURE FORMULA �PRESSURE = FORCE AREA = NEWTONS AREA = _N_ m 2

BUOYANT FORCE � THE DIFFERENCE IN PRESSURE RESULTS IN UPWARD FORCE ON AN OBJECT IN A FLUID.

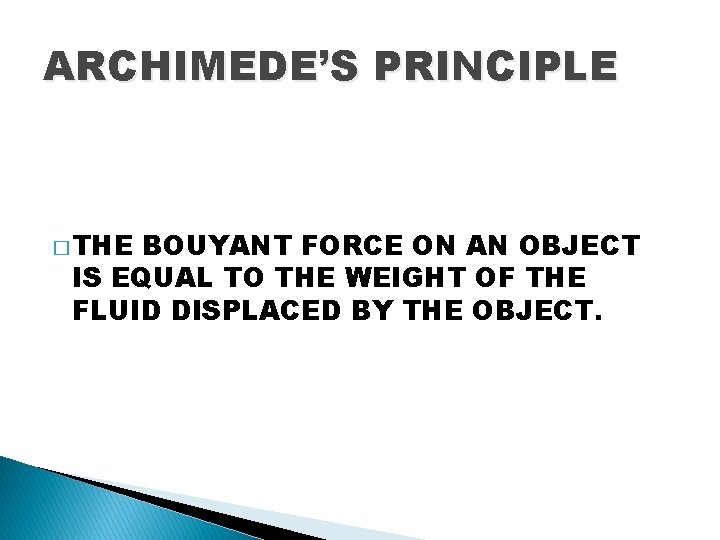
ARCHIMEDE’S PRINCIPLE � THE BOUYANT FORCE ON AN OBJECT IS EQUAL TO THE WEIGHT OF THE FLUID DISPLACED BY THE OBJECT.

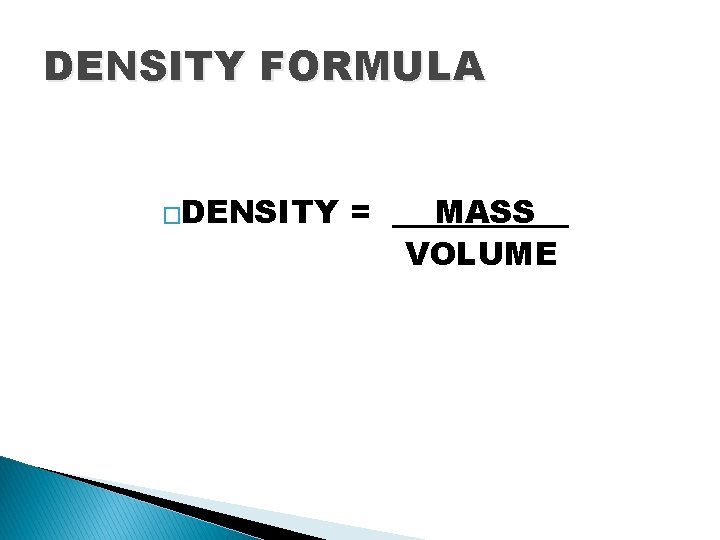
DENSITY FORMULA �DENSITY = __ MASS__ VOLUME

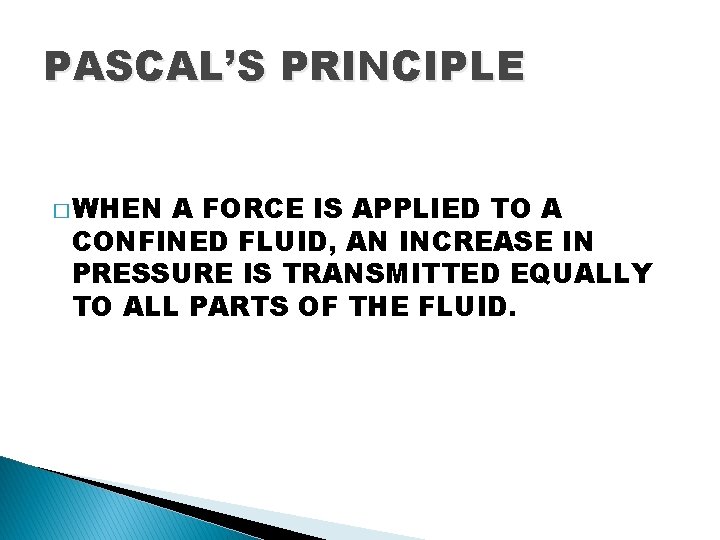
PASCAL’S PRINCIPLE � WHEN A FORCE IS APPLIED TO A CONFINED FLUID, AN INCREASE IN PRESSURE IS TRANSMITTED EQUALLY TO ALL PARTS OF THE FLUID.

QUESTIONS? ? ?