Matter Vocabulary matter anything that has mass takes

Matter Vocabulary

matter _________ anything that has mass takes up space

_________ property describes an object (color, size, texture)
atom _________ small particle that is the basic unit of matter
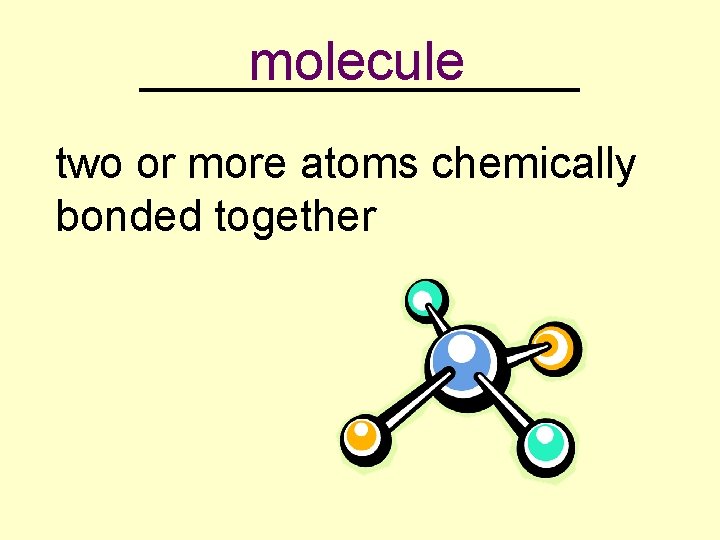
molecule _________ two or more atoms chemically bonded together
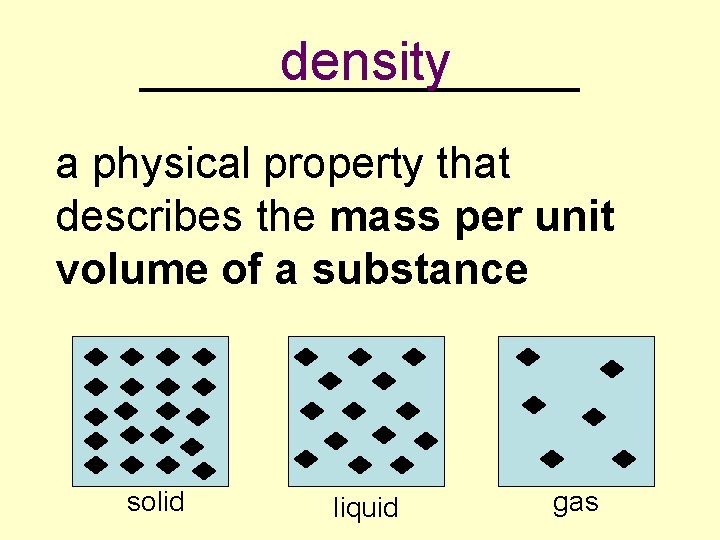
density _________ a physical property that describes the mass per unit volume of a substance solid liquid gas
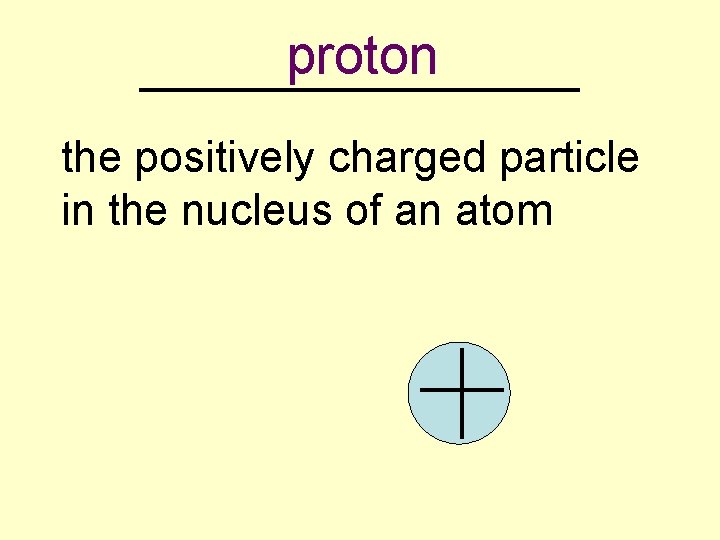
proton _________ the positively charged particle in the nucleus of an atom
neutron _________ a particle with no charge in the nucleus of an atom
electron _________ negatively charged particles in an atom (in a cloud around the nucleus)

element _________ matter made of only one type of atom Examples: oxygen, calcium
Periodic Table _________ a chart that organizes all known elements

_________ compound matter made by combining two or more different kinds of elements Examples: water
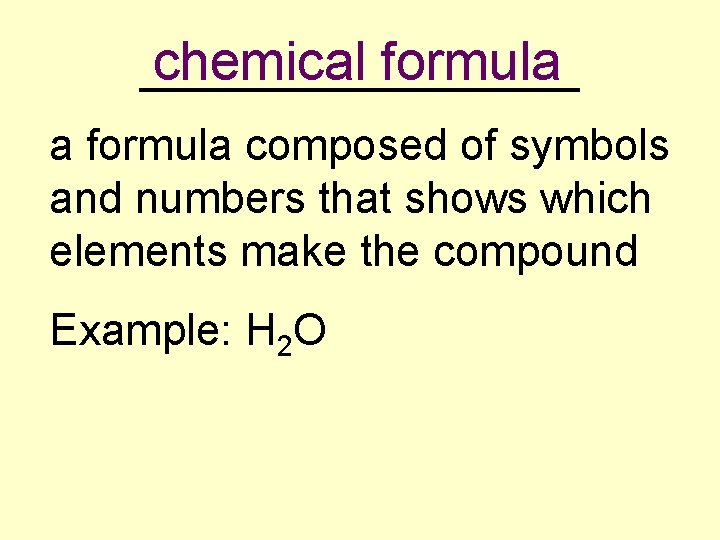
chemical formula _________ a formula composed of symbols and numbers that shows which elements make the compound Example: H 2 O

mixture _________ two or more substances put together that do not form a compound Example: rocks, air
mass _________ a measure of the amount of matter in an object
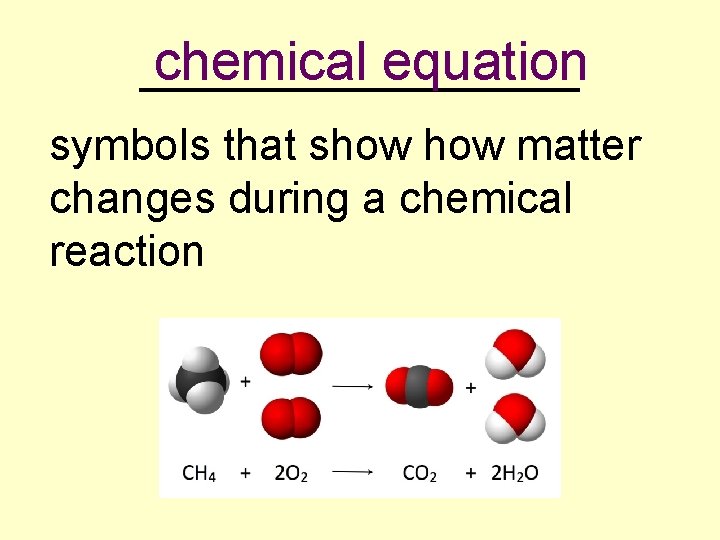
chemical equation _________ symbols that show matter changes during a chemical reaction
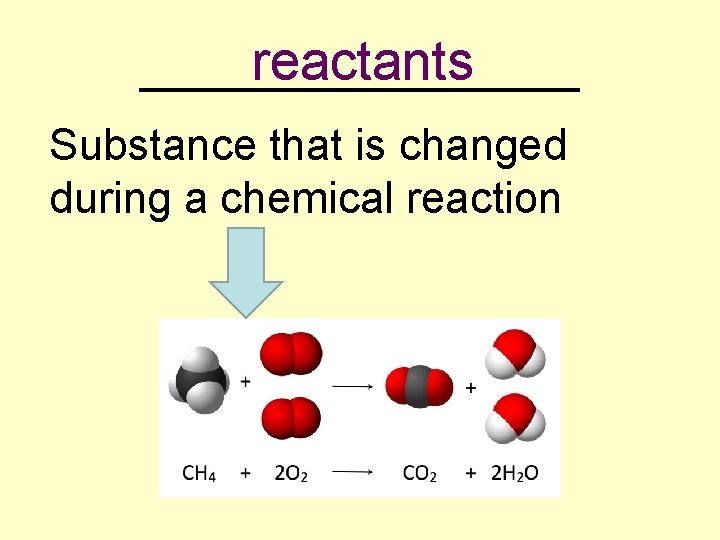
reactants _________ Substance that is changed during a chemical reaction
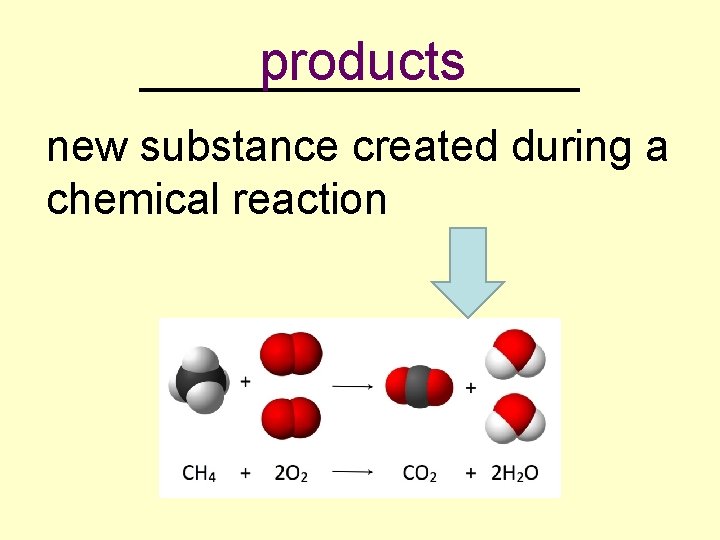
products _________ new substance created during a chemical reaction
- Slides: 18





























