Matter Anything that has mass and takes up




- Slides: 22


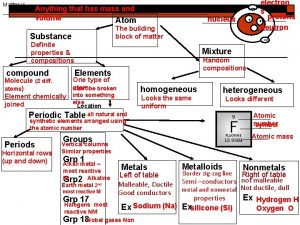
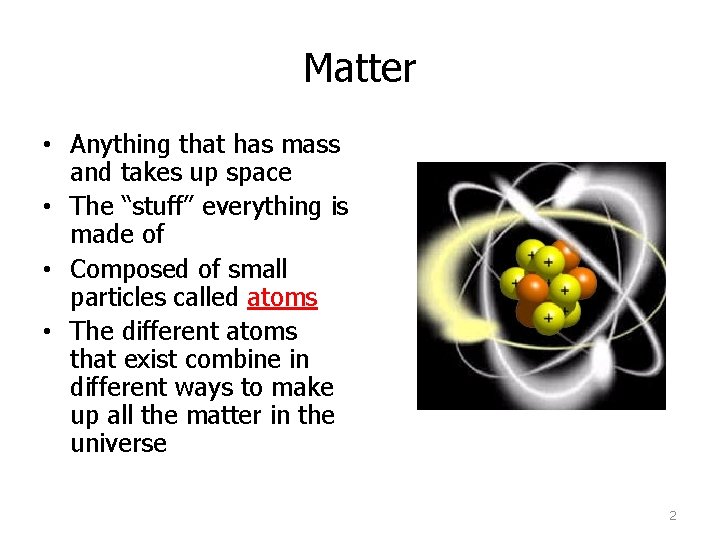
Matter • Anything that has mass and takes up space • The “stuff” everything is made of • Composed of small particles called atoms • The different atoms that exist combine in different ways to make up all the matter in the universe 2

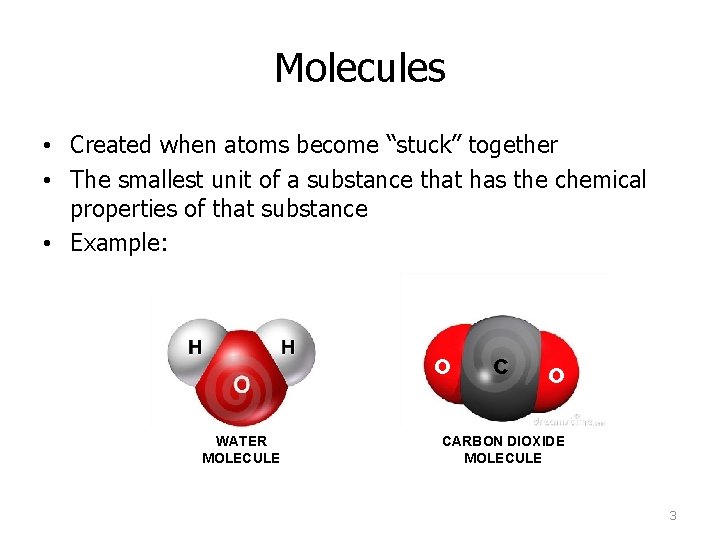
Molecules • Created when atoms become “stuck” together • The smallest unit of a substance that has the chemical properties of that substance • Example: o WATER MOLECULE c o CARBON DIOXIDE MOLECULE 3

Elements • Substances containing only one type of atom – Cl 2 – N 2 – H 2 Remember: Diatomic Elements Br I N Cl H O F • Found on the periodic table • Cannot be broken down into simpler substances by chemical means 4

Compounds • Substances containing 2 or more different types of atoms combined in a definite ratio • Example: – H 2 O (2 atoms hydrogen; 1 atom oxygen) – Na. Cl (1 atom sodium; 1 atom chlorine) – CO 2 (1 atom carbon; 2 atoms oxygen) • Can be broken down by chemical means 5

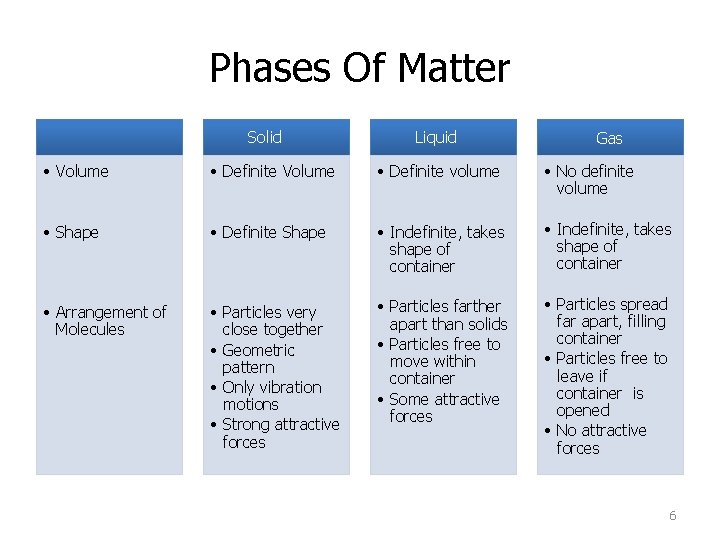
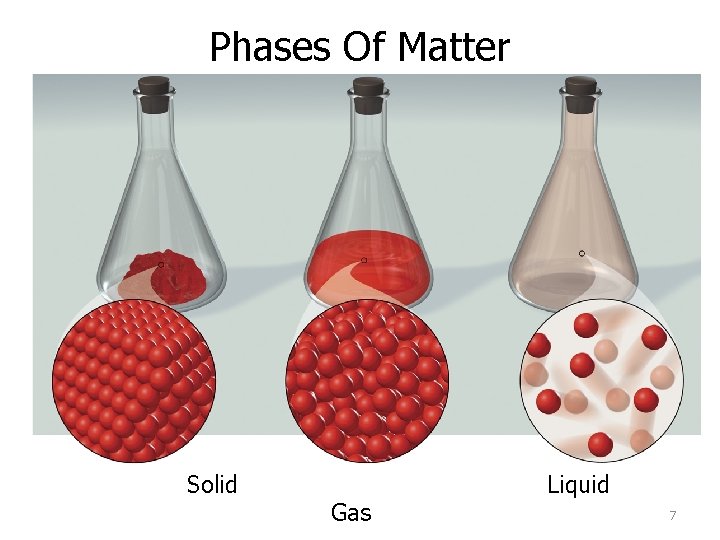
Phases Of Matter Solid Liquid Gas • Volume • Definite volume • No definite volume • Shape • Definite Shape • Indefinite, takes shape of container • Arrangement of Molecules • Particles very close together • Geometric pattern • Only vibration motions • Strong attractive forces • Particles farther apart than solids • Particles free to move within container • Some attractive forces • Particles spread far apart, filling container • Particles free to leave if container is opened • No attractive forces 6

Phases Of Matter Solid Gas Liquid 7

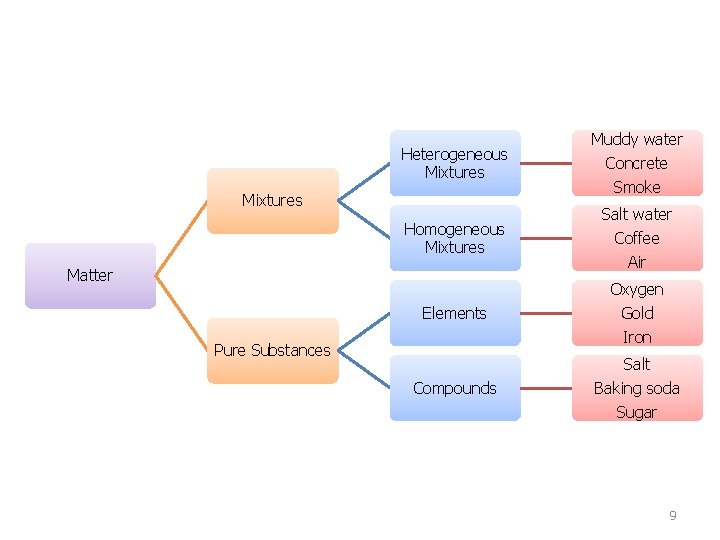
Mixture • Contains two or more components not chemically combined 1. Homogeneous • • • Same throughout; uniformly mixed Also known as solutions Ex. Air (gas), milk (liquid), brass (solid) 2. Heterogeneous • • Regions differ from one area to another; not uniformly mixed Ex. Chocolate chip cookie, Italian salad dressing 8

Heterogeneous Mixtures Homogeneous Mixtures Matter Muddy water Concrete Smoke Salt water Coffee Air Oxygen Elements Gold Iron Pure Substances Salt Compounds Baking soda Sugar 9

Separating Mixtures • By physical means such as: – Decanting • Separates a liquid from insoluble solid sediment • Involves carefully pouring the liquid from the solid without disturbing the solid 10

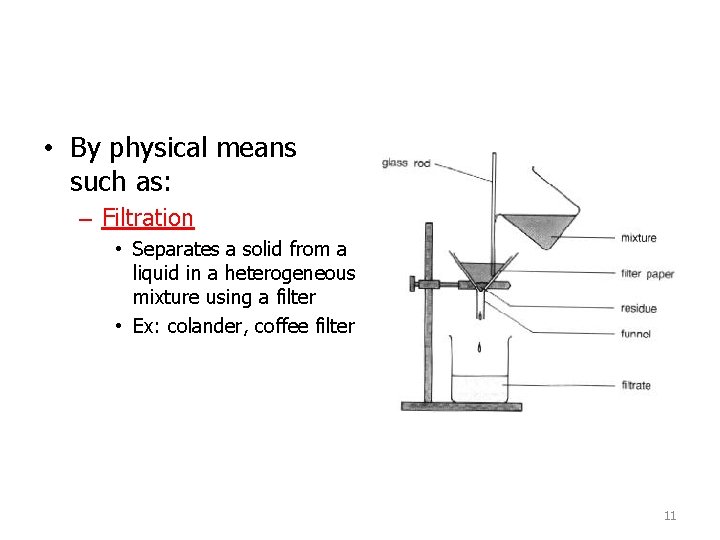
• By physical means such as: – Filtration • Separates a solid from a liquid in a heterogeneous mixture using a filter • Ex: colander, coffee filter 11

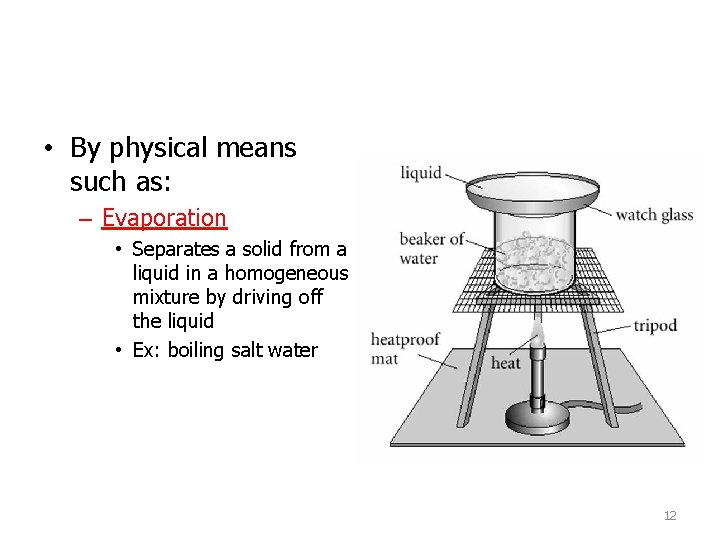
• By physical means such as: – Evaporation • Separates a solid from a liquid in a homogeneous mixture by driving off the liquid • Ex: boiling salt water 12

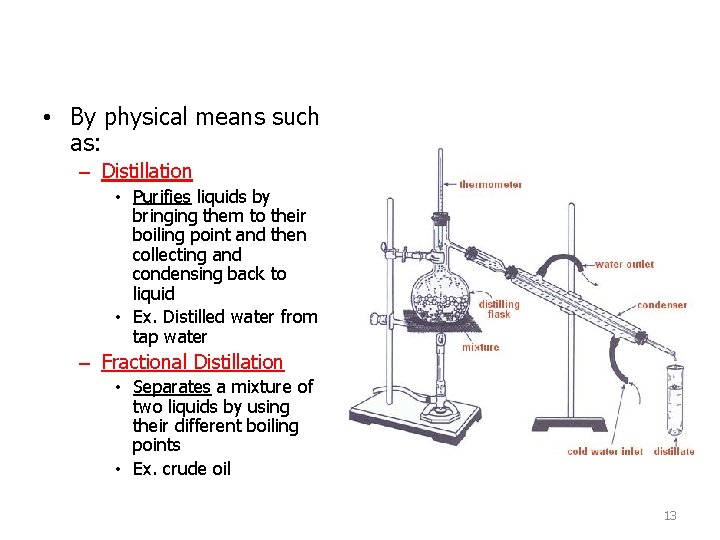
• By physical means such as: – Distillation • Purifies liquids by bringing them to their boiling point and then collecting and condensing back to liquid • Ex. Distilled water from tap water – Fractional Distillation • Separates a mixture of two liquids by using their different boiling points • Ex. crude oil 13

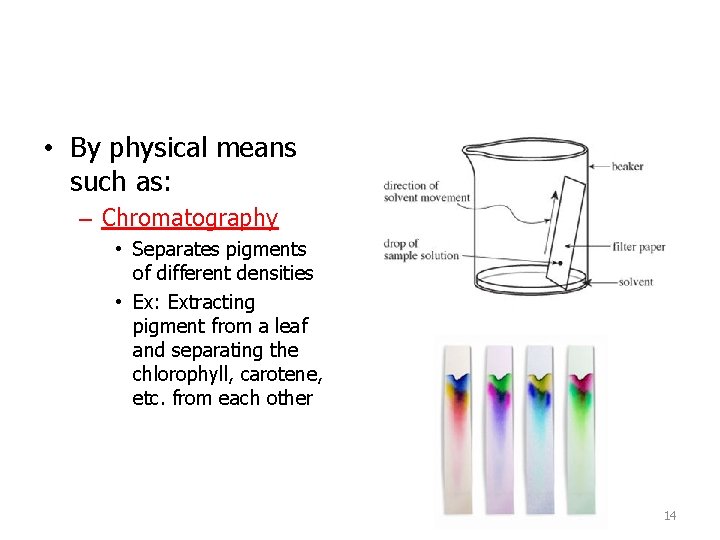
• By physical means such as: – Chromatography • Separates pigments of different densities • Ex: Extracting pigment from a leaf and separating the chlorophyll, carotene, etc. from each other 14

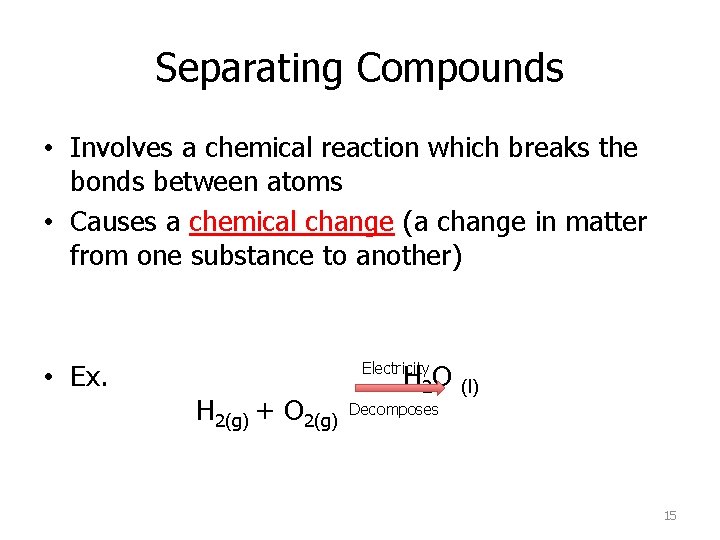
Separating Compounds • Involves a chemical reaction which breaks the bonds between atoms • Causes a chemical change (a change in matter from one substance to another) • Ex. H 2 O Electricity H 2(g) + O 2(g) (l) Decomposes 15

Try These!!! 16

Properties • Used to describe substances (matter that has a uniform and definite composition) Physical Properties • Properties that can change without the substance becoming a new substance Chemical Properties • Properties that cause a substance to become a new substance 17

Intensive Physical Properties (depend on the type of matter) • • State (phase) of matter, color, odor, density, solubility, hardness, melting point, boiling point Malleability Capable of being hammered into sheets – • • Ductility Capable of being drawn into wire – • • Aluminum foil Copper Conductivity – – Ability of electrons to flow Heat and electrical 18

Extensive Physical Properties (depend on the amount of matter) • Volume – Space occupied by an object • Mass – Amount of matter an object contains 19

Chemical Properties • Rusting • Burning • Leaves turning color • Digestion 20

Physical & Chemical Changes Physical Changes • Change in form or phase of a substance but not its chemical composition • Ex. – Phase changes Chemical Changes • • Melting, freezing, evaporation, condensation, sublimation – Cutting hair – Breaking a window – Crushing a piece of chalk Change in chemical composition due to a reaction Ex. – Silver tarnishes by reacting with substances in the air – Making scrambled eggs – Burning a candle Evidence of a chemical change may include: – – • Transfer of energy Change in color Production of gas Formation of a precipitate (solid that forms and settles out of a liquid mixture) Referred to as chemical reactions 21

Chemical Reactions • When at least 1 substance changes into a new substance Z (products) X + Y (reactants) • Reactant- Substances present at the start of a reaction • Product- Substances present at the end of a reaction 22
 Anything which occupies space and has mass
Anything which occupies space and has mass Is anything that has mass and takes up space.
Is anything that has mass and takes up space. Matter anything that takes up space
Matter anything that takes up space Solubility defintion
Solubility defintion Anything that has mass and takes up space
Anything that has mass and takes up space Matter is anything that has
Matter is anything that has What are the 7 diatomic elements
What are the 7 diatomic elements Whats anything that has mass and takes up space
Whats anything that has mass and takes up space A glass sinker has a mass m in air
A glass sinker has a mass m in air Whats anything that has mass and takes up space
Whats anything that has mass and takes up space Anything that takes up space and has mass
Anything that takes up space and has mass Matter is anything that has mass and volume.
Matter is anything that has mass and volume. Matter is anything
Matter is anything Matter is anything that occupies space
Matter is anything that occupies space It is anything that has mass and occupies space
It is anything that has mass and occupies space It is anything that has mass and occupies space
It is anything that has mass and occupies space Phân độ lown
Phân độ lown Block nhĩ thất độ 3
Block nhĩ thất độ 3 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của đường thẳng
Tìm vết của đường thẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều