Matter Anything that has mass and occupies space











- Slides: 14

Matter • Anything that has mass and occupies space • Law of Conservation of Matter and Energy • Matter and energy can neither be created or destroyed but can change form • Mass • Measure of the quantity of matter • Material • Describes a specific type of matter ex. Wood, plastic, water

• Mixture –Matter that contains two or more different materials • Ex. Wood, air, milk • Characteristics of a Mixture • Can be prepared and separated by physical change • Substance composing a mixture retain some properties when separate • Substances can be in any proportions • Can be homogenous or heterogeneous

• Phase • Physically distinct section of matter with a uniform set of properties • Ex. Water in milk and fat in milk

• State • Particle arrangement in a phase • Solid, liquid, gas, plasma • Interfaces • Definite boundaries between phases of matter in a mixture • Ex. Solid surface of ice meets surface of liquid water

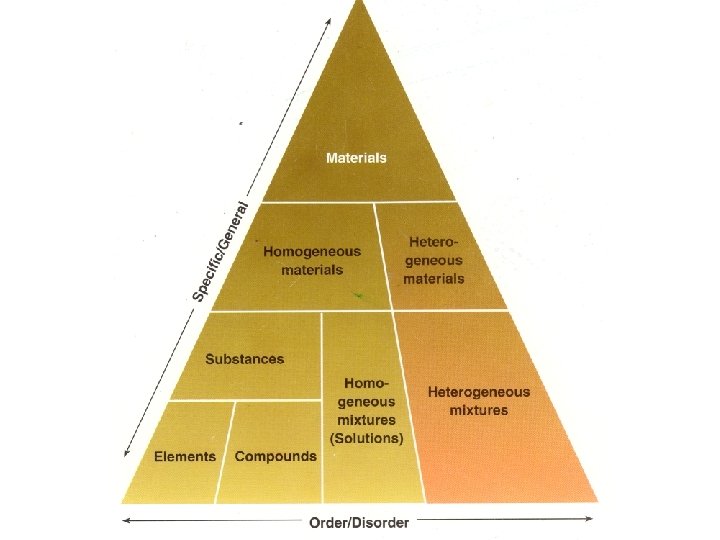
Heterogeneous materials • Nonuniform materials • Materials are easily separated and distinguished from each other • Heterogeneous mixtures • Contain 2 or more materials nonuniformly distributed throughout the mixture • Ex. Sand, salt and iron particles

Homogenous materials • Materials that consist of only one phase • All parts have the same properties • Impossible to distinguish one part as a different material from another • Ex. Sugar, salt, glass, quartz, air • Homogeneous mixtures • 2 or more materials combined and uniformly distributed throughout the mixture • Called solutions

Separation of mixtures • Filtration • Uses a porous barrier to separate solids from liquids • Distillation • Allows separation of homogenous mixtures of liquids based on B. P. differences • Crystallization • formation of a pure solid substance from a solution containing a dissolved substance • Chromatography • Technique that separates 2 or more dissolved solids on the basis that they travel at different rates across a different material

Substances • Homogenous material with the same composition throughout • Two classes • Compounds • Two or more atoms(elements) chemically combined in definite proportions(ratios) • Chemical and Physical properties of elements in a compound are different than their constituents • Organic(contain C in structure) and inorganic(compounds without C) • Ex. Na. Cl, C 6 H 12 O 6, H 2 O • Elements • Substances comprised of only one type of atom • Free state and combined state • Ex. Fe, Na, O, N


Changes and Properties • Physical change • A change which does not change the chemical composition of the substance • Ex. Changes in state, dissolving, cutting

Chemical • Describes the reaction of a substance with another material • Just as important to find out if a substance does react as it does not react • Ex. Flammability, toxicity • How reacts with : air, water, acid, base

Properties • Physical • Describes the behavior of a substance undergoing a physical change • Two types • Extensive- depends on the amount of matter present • Ex. Mass, weight, volume

• Intensive – do not depend on amount of substance present • Useful for identifying substances • Ex. Density • Unique to every substance • Mass/volume • Specific heat, malleability, ductility, color, crystalline shape, melting point (M. P. ), boiling point (B. P. )

• Chemical • When a substance undergoes a change so that one or more new substances with different characteristics are formed • When a chemical reaction takes place • Ex. Burning, oxidation, fermentation • Rule of thumb: if a precipitate, gas, color change, or energy change occurs, a chemical change has usually taken place • Precipitate- a solid substance that forms from a solution
 Matter is anything that has mass and occupies space.
Matter is anything that has mass and occupies space. Matter is anything that...
Matter is anything that... The matter is anything that occupies
The matter is anything that occupies It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter has mass and occupies space
Matter has mass and occupies space All matter has and takes up
All matter has and takes up Matter is anything that has mass and volume.
Matter is anything that has mass and volume. Mass vs weight
Mass vs weight Matter is anything that has mass
Matter is anything that has mass Anything that has mass
Anything that has mass Matter is anything that has and takes up
Matter is anything that has and takes up Anything that has mass and takes up space
Anything that has mass and takes up space Matter is anything that has both
Matter is anything that has both Celsius degree symbol
Celsius degree symbol Anything that takes up space and has mass
Anything that takes up space and has mass

























