Anything that has occupies mass space Mass Weight








- Slides: 17

Anything that has occupies mass & space

Mass Weight • Stays Constant • Changes with location • Measures quantity of matter • Measures the pull of gravity (which changes) on an object • Unit is kilogram (kg) or gram (g) • Unit is Newton (N)

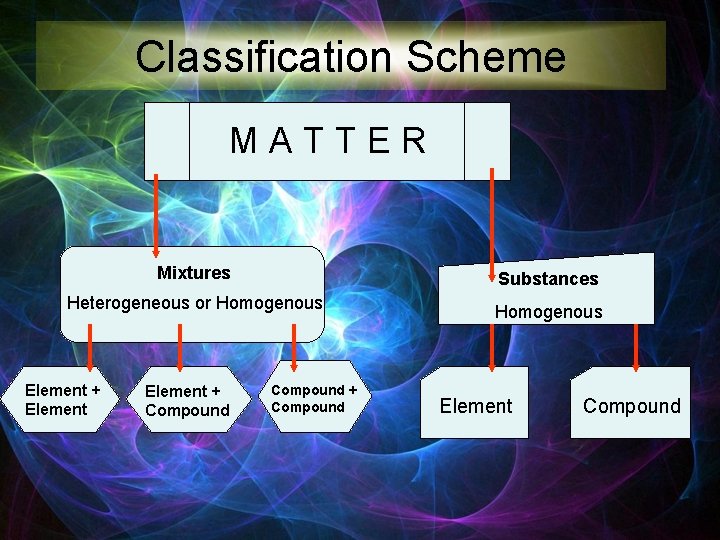
Classification All matter can be classified into two groups ØSubstances ØMixtures

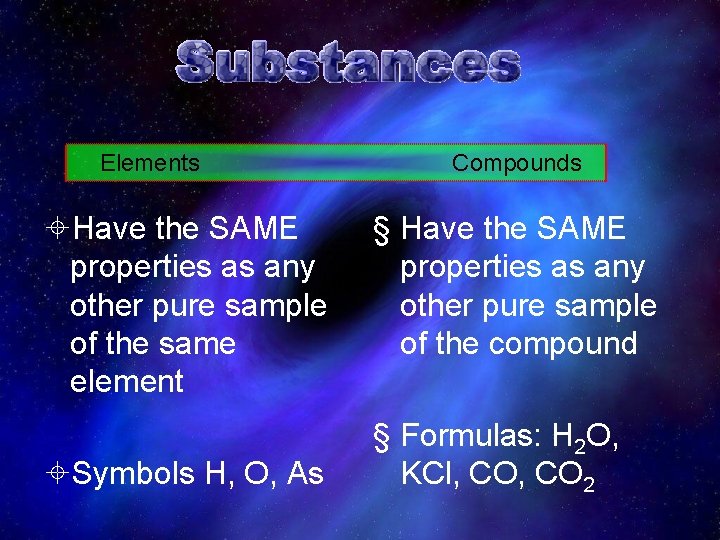
¤ Elements – Cannot be broken down into other substances by ordinary chemical change – 112 elements – Many atoms of one type=Element ¤ Compounds – Elements in compound are combined in definite proportions • Always the SAME proportion for any given compound – The compound has new properties different from the elements that make it up.

Elements ±Have the SAME properties as any other pure sample of the same element ±Symbols H, O, As Compounds § Have the SAME properties as any other pure sample of the compound § Formulas: H 2 O, KCl, CO 2

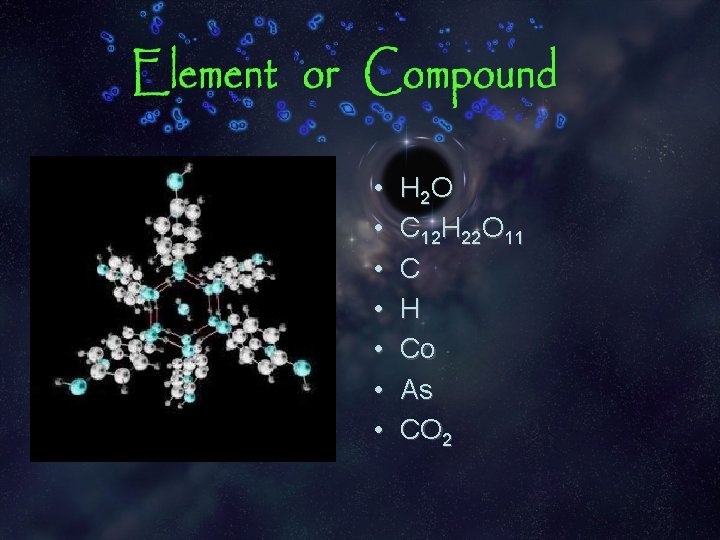
• • H 2 O C 12 H 22 O 11 C H Co As CO 2

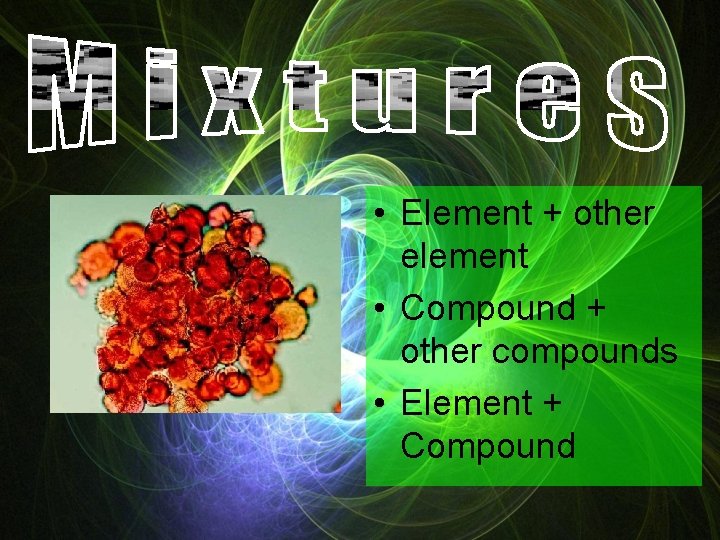
Consist of 2 or more substances that retain their individual properties

• Homogenous – The mixture has the same composition throughout. – Ex: Coffee is mixed thoroughly so that it is the same strength everywhere • Heterogeneous – The mixture has a different composition throughout. It is unevenly mixed. – Ex. : Trail mix – you can pick out the raisins if you don’t want them.

• Only one set of properties • Composition always fixed – ex: H 2 O or Cl 2 • Always HOMOGENEOUS • Each constituent keeps it own properties – ex: sugar mixed in H 20 • Composition varies e. g. very sweet or a little sweet • May be Homogeneous or Heterogeneous

• Element + other element • Compound + other compounds • Element + Compound

Classification Scheme MATTER Mixtures Substances Heterogeneous or Homogenous Element + Compound + Compound Element Compound

Element-Compound-Mixture? • H 2 SO 4 • Steel girders – Steel = alloy • As (Arsenic) – Alloy is a solid • Aqueous (watersolution of 2 or more based) Na. Cl solution metals • Sand + H 2 O • C 2 H 5 OH • Zinc washers (pure Zn)

Chemical Formulas How to interpret them and what they mean!

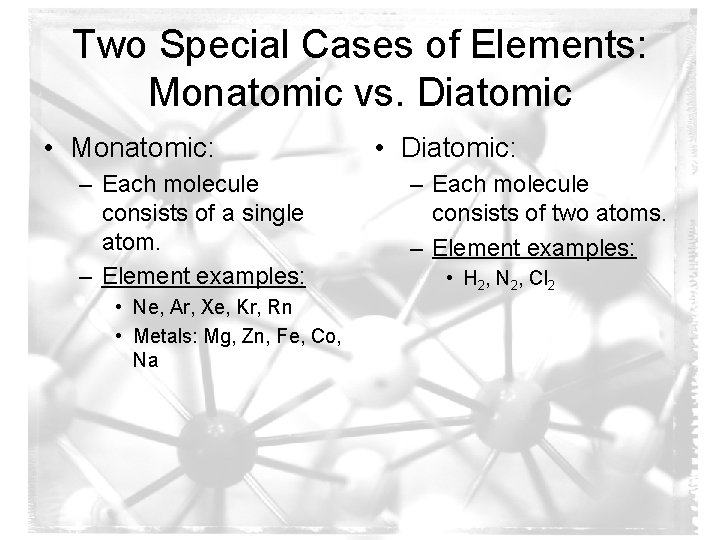
Two Special Cases of Elements: Monatomic vs. Diatomic • Monatomic: – Each molecule consists of a single atom. – Element examples: • Ne, Ar, Xe, Kr, Rn • Metals: Mg, Zn, Fe, Co, Na • Diatomic: – Each molecule consists of two atoms. – Element examples: • H 2, N 2, Cl 2

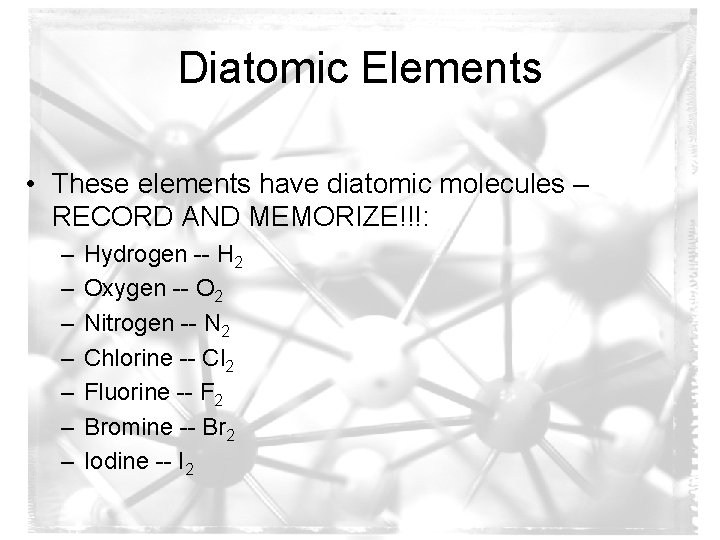
Diatomic Elements • These elements have diatomic molecules – RECORD AND MEMORIZE!!!: – – – – Hydrogen -- H 2 Oxygen -- O 2 Nitrogen -- N 2 Chlorine -- Cl 2 Fluorine -- F 2 Bromine -- Br 2 Iodine -- I 2

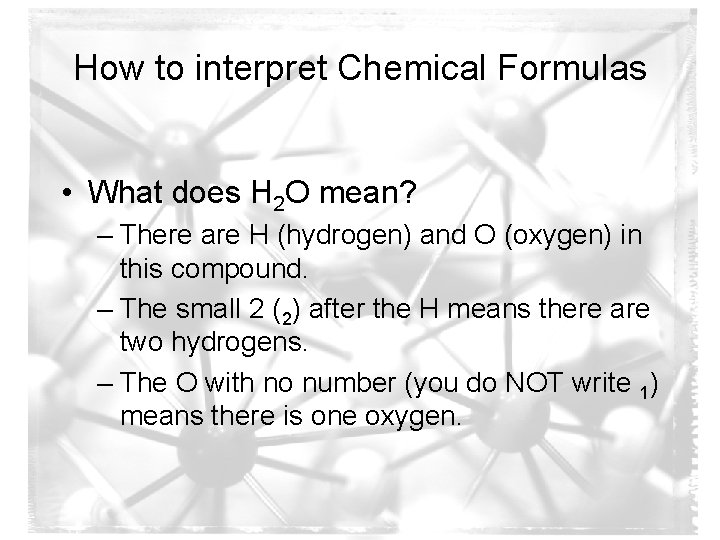
How to interpret Chemical Formulas • What does H 2 O mean? – There are H (hydrogen) and O (oxygen) in this compound. – The small 2 (2) after the H means there are two hydrogens. – The O with no number (you do NOT write 1) means there is one oxygen.

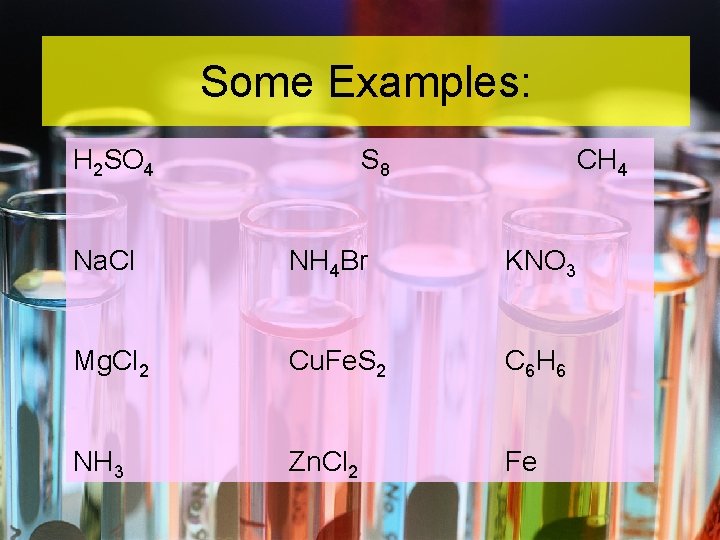
Some Examples: H 2 SO 4 S 8 CH 4 Na. Cl NH 4 Br KNO 3 Mg. Cl 2 Cu. Fe. S 2 C 6 H 6 NH 3 Zn. Cl 2 Fe