Matter Anything that has mass and takes up








- Slides: 21

Matter Anything that has mass and takes up space (volume) What is NOT matter? ? ?

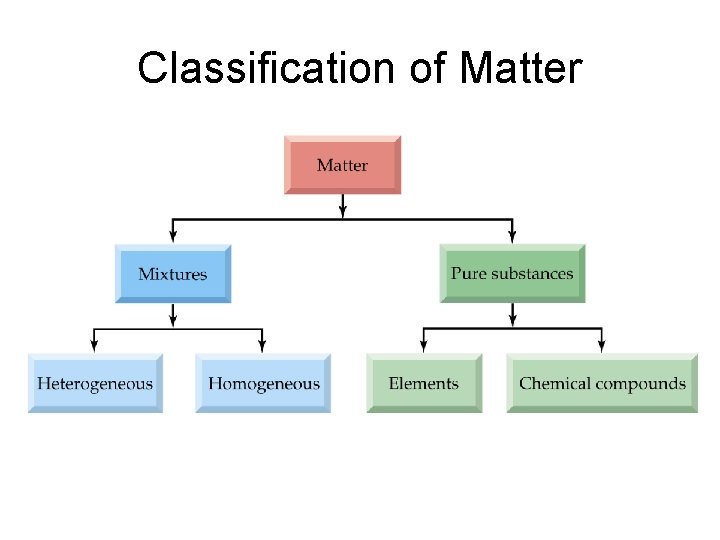
Classification of Matter

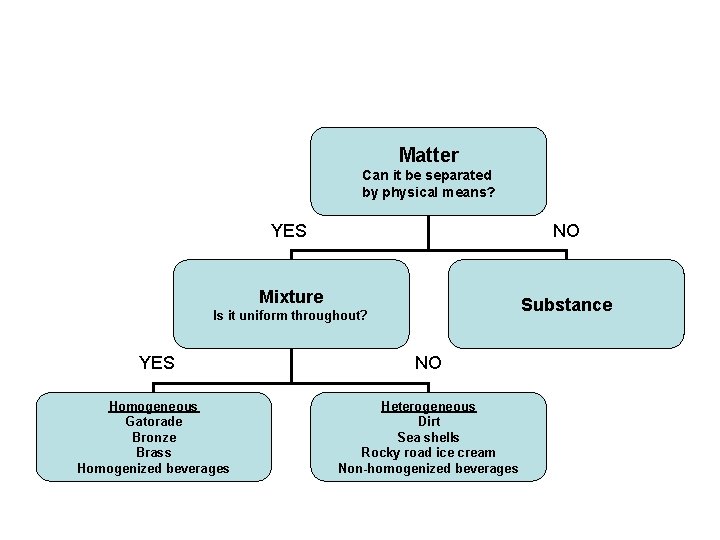
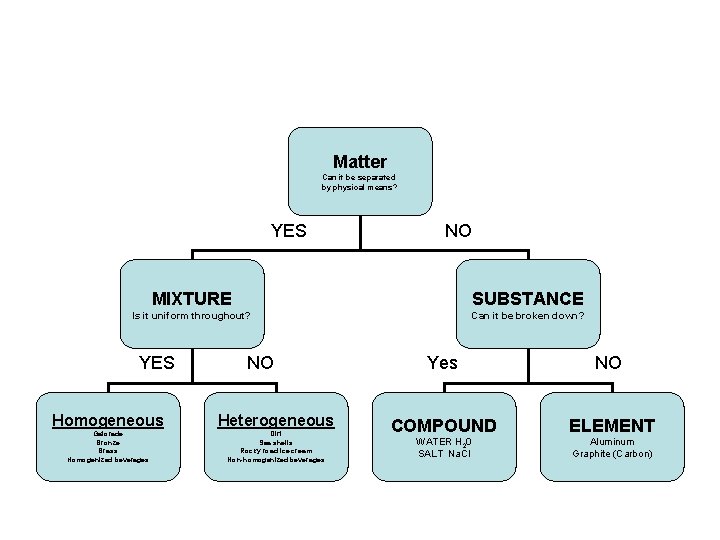
Matter Can it be separated by physical means? If yes….

Then you have a Mixture

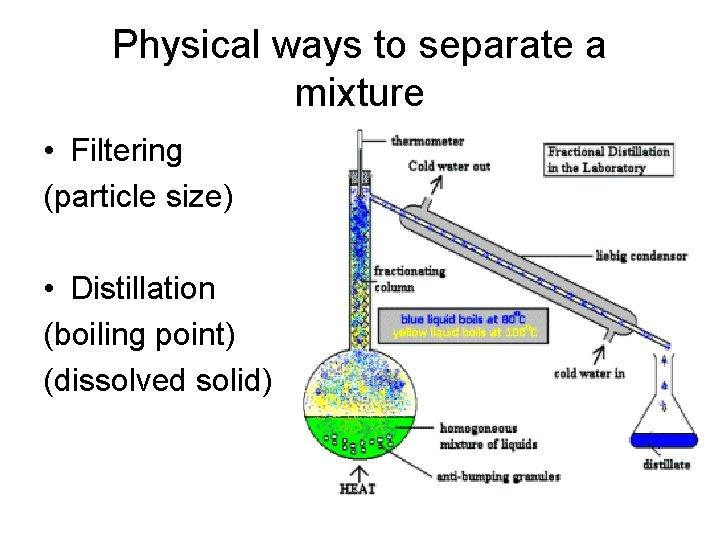
Physical ways to separate a mixture • Filtering (particle size) • Distillation (boiling point) (dissolved solid)

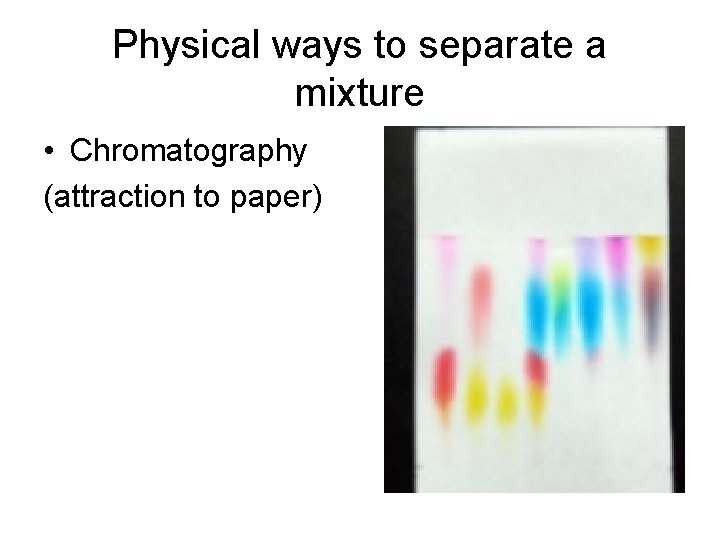
Physical ways to separate a mixture • Chromatography (attraction to paper)

Is the mixture uniform throughout? • IF yes, it is HOMOGENEOUS

If no, then the mixture is Heterogeneous

Homogeneous vs. Heterogeneous mixtures

Matter Can it be separated by physical means? YES NO Mixture Substance Is it uniform throughout? YES NO Homogeneous Gatorade Bronze Brass Homogenized beverages Heterogeneous Dirt Sea shells Rocky road ice cream Non-homogenized beverages

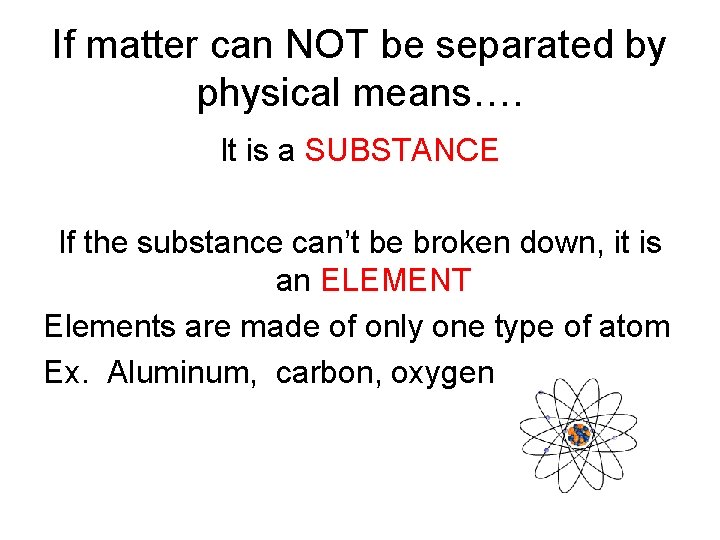
If matter can NOT be separated by physical means…. It is a SUBSTANCE If the substance can’t be broken down, it is an ELEMENT Elements are made of only one type of atom Ex. Aluminum, carbon, oxygen

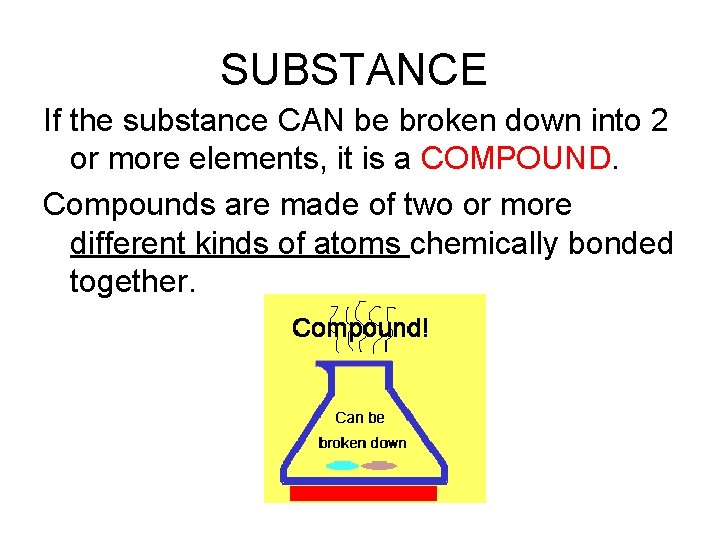
SUBSTANCE If the substance CAN be broken down into 2 or more elements, it is a COMPOUND. Compounds are made of two or more different kinds of atoms chemically bonded together.

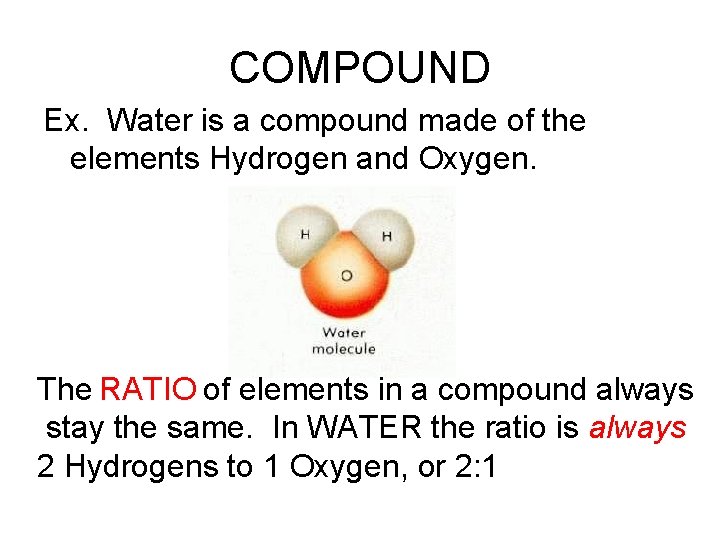
COMPOUND Ex. Water is a compound made of the elements Hydrogen and Oxygen. The RATIO of elements in a compound always stay the same. In WATER the ratio is always 2 Hydrogens to 1 Oxygen, or 2: 1

Matter Can it be separated by physical means? YES NO MIXTURE SUBSTANCE Is it uniform throughout? Can it be broken down? YES NO Homogeneous Heterogeneous Gatorade Bronze Brass Homogenized beverages Dirt Sea shells Rocky road ice cream Non-homogenized beverages Yes NO COMPOUND ELEMENT WATER H 20 SALT Na. Cl Aluminum Graphite (Carbon)

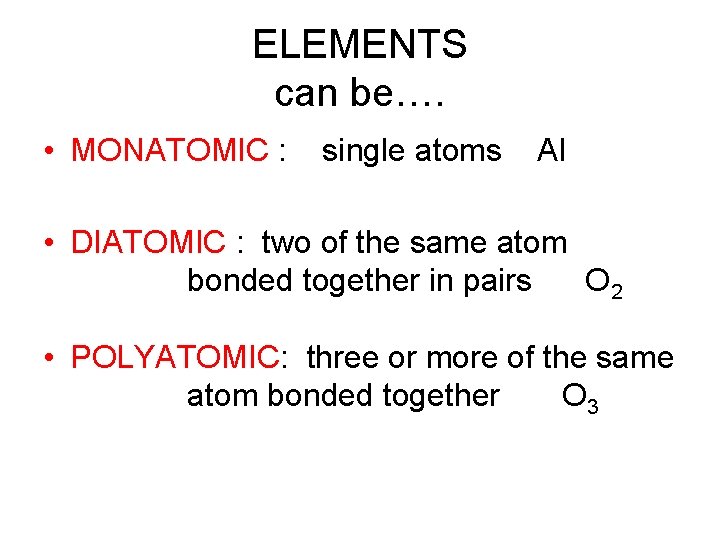
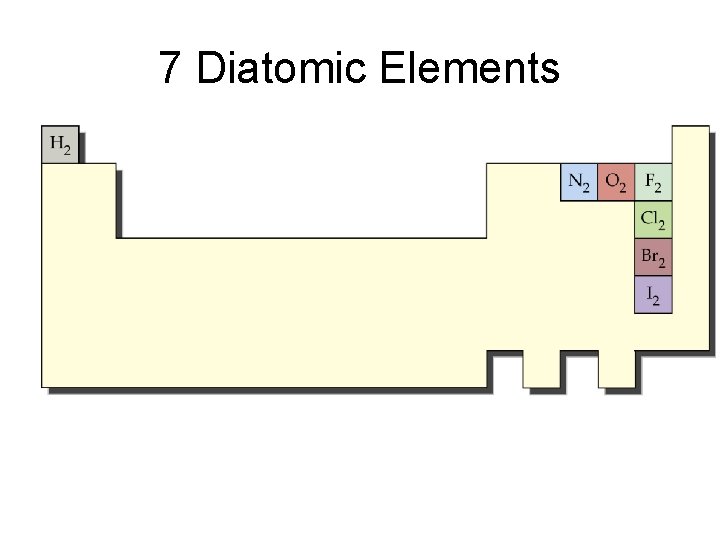
ELEMENTS can be…. • MONATOMIC : single atoms Al • DIATOMIC : two of the same atom bonded together in pairs O 2 • POLYATOMIC: three or more of the same atom bonded together O 3

7 Diatomic Elements

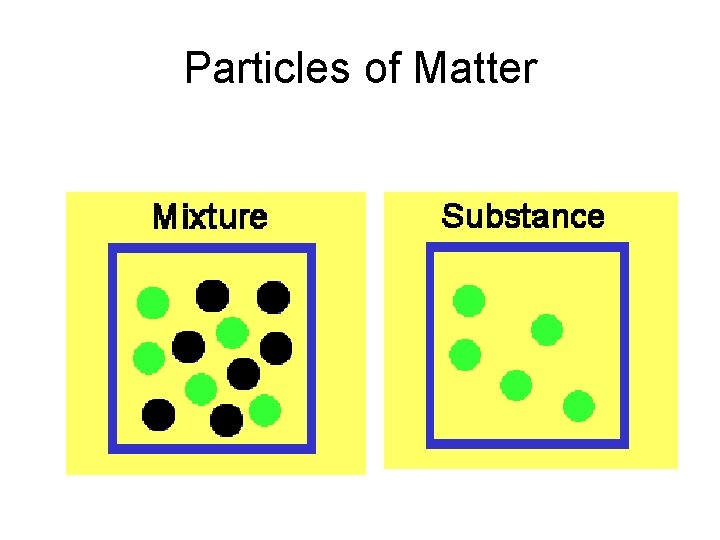
Particles of Matter

Review What is…… MATTER SUBSTANCE MIXTURE ELEMENT COMPOUND HETEROGENEOUS VS. HOMOGENEOUS

CHEMISTRY: THE STUDY OF MATTER Did you know that……. . One of the most popular “foods” in the United States is an entirely SYNTHETIC (man-made) mixture. Can you guess what it is?

SODA The average person consumes 40 GALLONS (!) of soda each year Mixture: carbonated water sweetener, acids, flavorings, caffeine

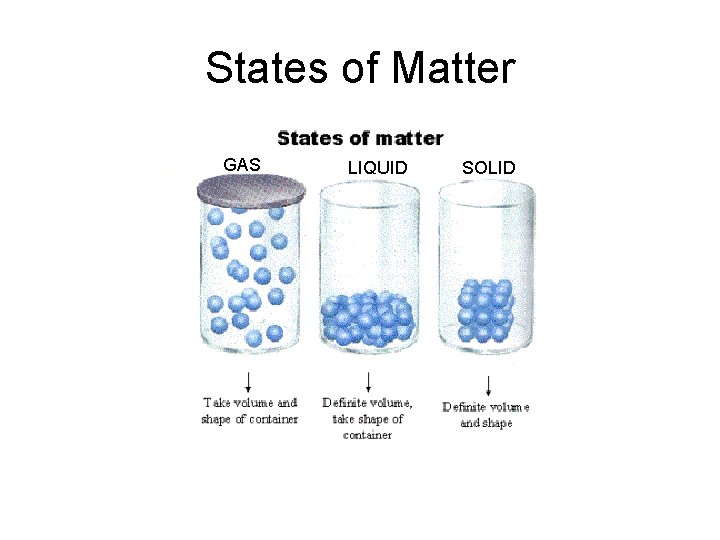
States of Matter GAS LIQUID SOLID