Matter anything that has mass and takes up












- Slides: 18

Matter: anything that has mass and takes up space Mass: the amount of matter an object has Weight: the force applied to an object by gravity Substance: a sample that contains only one kind of matter

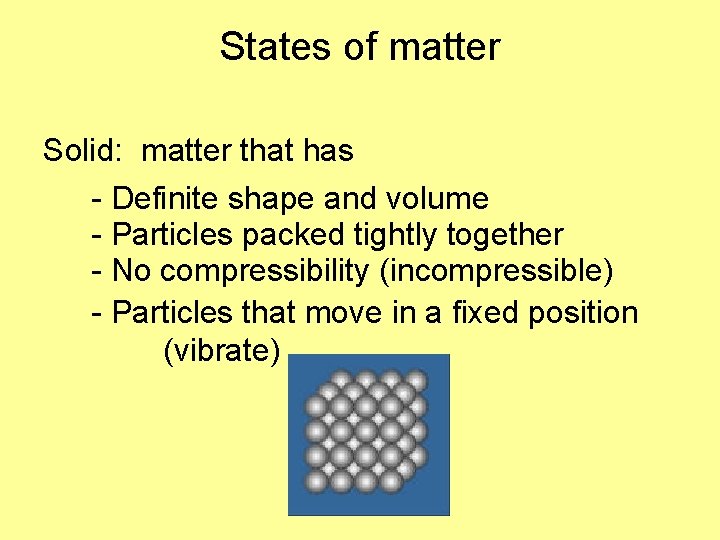
States of matter Solid: matter that has - Definite shape and volume - Particles packed tightly together - No compressibility (incompressible) - Particles that move in a fixed position (vibrate)

Liquids: matter that - Particles are close but not in a fixed position - Flows - Takes the shape of its container - Has a definite volume - No compressibility (incompressible)

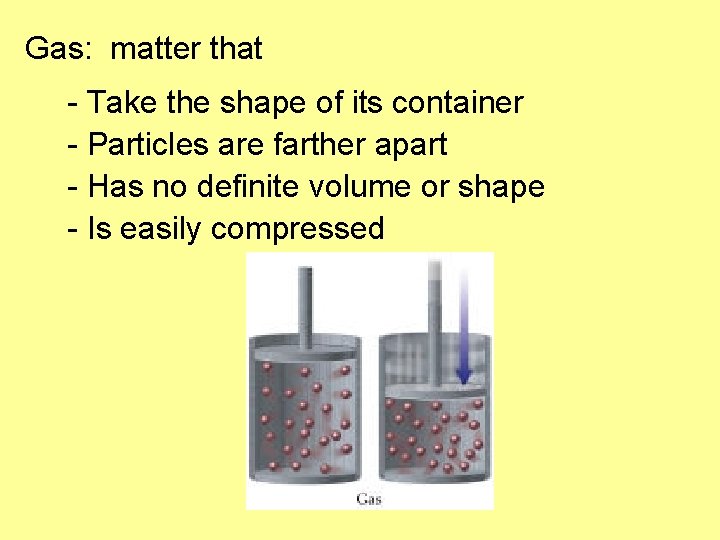
Gas: matter that - Take the shape of its container - Particles are farther apart - Has no definite volume or shape - Is easily compressed

Physical Property: a property that can be observed or measured without changing the substance’s composition Ex: Color, Temperature Density Odor Hardness State of matter

Physical Change: a change in a substance which does not alter its chemical composition - Freezing - Melting - Boiling - Cutting - Grinding - Crumpling

Chemical Property: the ability of a substance to react and form a new substance (observed only when there is a chemical change) (most chemical changes are not easily reversed) Ex. Burning, rusting, rotting, corroding

Chemical Reactions Chemical Reaction: one or more substances change into a new substance Ex. Iron and oxygen react or make rust Reactants: the starting substances in a chemical reaction Products: What you end up with in a chemical reaction HCl + Na. OH → Na. Cl + H 2 O

How do you know if a chemical reaction is happening? There are four indications of a chemical reaction. (not all indicators will be easy to see during every chemical reaction) 1. 2. 3. 4. The production of a gas A change in energy A change in color The production of a precipitate

Mixtures: a physical blend of two or more substances Heterogeneous Mixtures: mixtures that are not uniform throughout or have more than one phase Ex. Oil and water, paint, chocolate chip cookies, and salad

Homogeneous Mixtures: a mixture that is uniform throughout and only has one phase Ex. Lemonade, Koolaid, and Salt water ALL MIXTURES CAN BE SEPERATED INTO THEIR INDIVIDUAL COMPONENTS!

Elements and Compounds Element: the simplest form of matter - Can NOT be separated into simpler substances through ordinary chemical means - They are the building blocks for all substances - They are represented by one or two letter symbols (C for Carbon, H for Hydrogen)

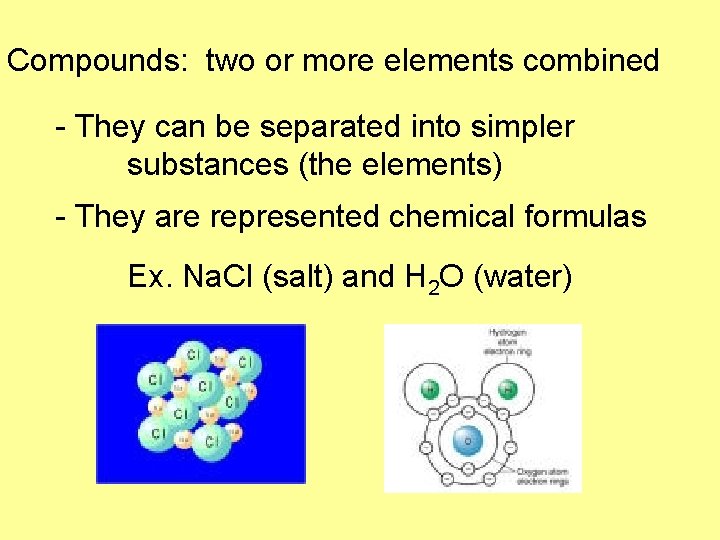
Compounds: two or more elements combined - They can be separated into simpler substances (the elements) - They are represented chemical formulas Ex. Na. Cl (salt) and H 2 O (water)

The Law of Conservation of Mass - Mass is never created nor destroyed in a chemical or physical change. - The mass of the products always equals the mass of the reactants.

Intensive Properties: properties that do not depend on the amount of matter present. Ex. Melting point Extensive Properties: properties that do depend on the amount of matter present Ex. mass

Qualitative and Quantitative Measurements Qualitative Measurement: descriptive, nonnumerical information Quantitative Measurement: a numerical measurement

How close is your measurement? Accuracy: How close your measurement is to the actual (accepted) measurement Precision: a measure of how close a series of measurements are to each other

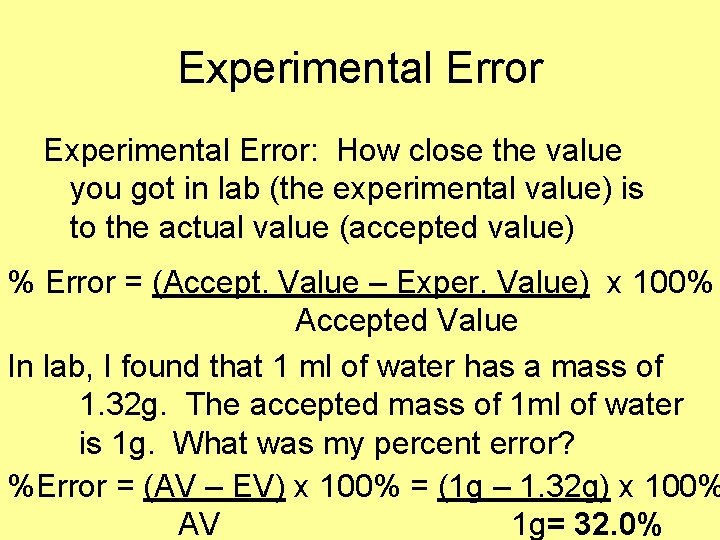
Experimental Error: How close the value you got in lab (the experimental value) is to the actual value (accepted value) % Error = (Accept. Value – Exper. Value) x 100% Accepted Value In lab, I found that 1 ml of water has a mass of 1. 32 g. The accepted mass of 1 ml of water is 1 g. What was my percent error? %Error = (AV – EV) x 100% = (1 g – 1. 32 g) x 100% AV 1 g= 32. 0%
 What is anything that has mass and occupies space
What is anything that has mass and occupies space Anything that takes up space and has mass is
Anything that takes up space and has mass is Anything that has matter and takes up space
Anything that has matter and takes up space Physical properties defintion
Physical properties defintion Anything that has mass and take up space
Anything that has mass and take up space Anything that has mass and takes up space
Anything that has mass and takes up space Anything that takes up space and has mass is
Anything that takes up space and has mass is Matter is anything that has both
Matter is anything that has both Matter is anything that has mass
Matter is anything that has mass Anything that has volume and mass
Anything that has volume and mass Anything that takes up space and has mass
Anything that takes up space and has mass Matter is anything with mass and volume
Matter is anything with mass and volume Matter is anything that...
Matter is anything that... Matter is anything that
Matter is anything that It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter has mass and occupies space
Matter has mass and occupies space Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Premature atrial contraction
Premature atrial contraction Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật






























