Matter is anything that has mass and takes









- Slides: 20


Matter is anything that has mass and takes up space. Matter is composed of very small particles called atoms. In nature, there are 92 different forms of matter that we call elements. Atoms by Brainpop

There are 11 elements of which most living things are composed. The most abundant element in the human body is oxygen. The second most abundant element in the human body is carbon, and in third place is hydrogen. Together, these three elements make up 92% of a human being.

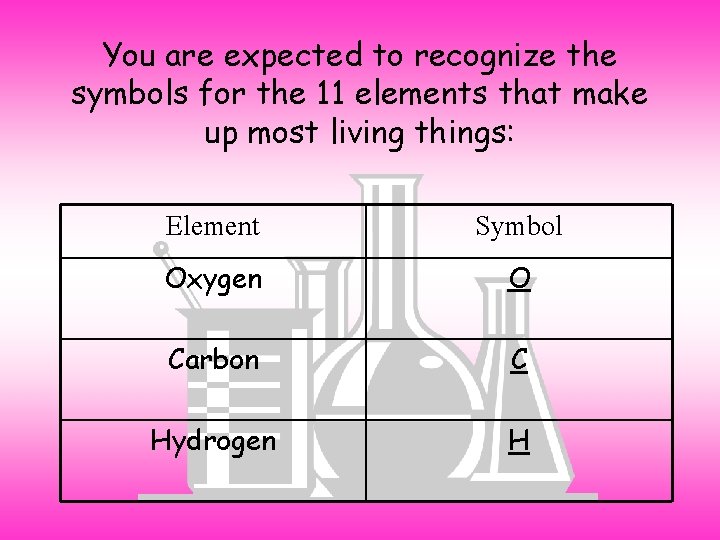
You are expected to recognize the symbols for the 11 elements that make up most living things: Element Symbol Oxygen O Carbon C Hydrogen H

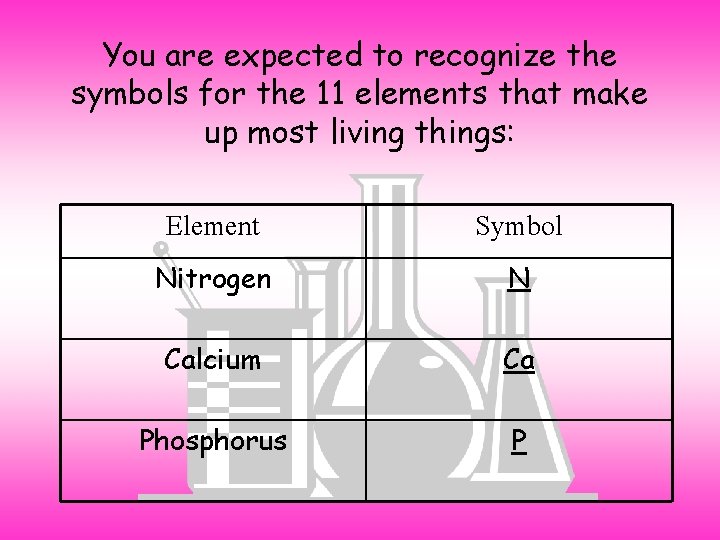
You are expected to recognize the symbols for the 11 elements that make up most living things: Element Symbol Nitrogen N Calcium Ca Phosphorus P

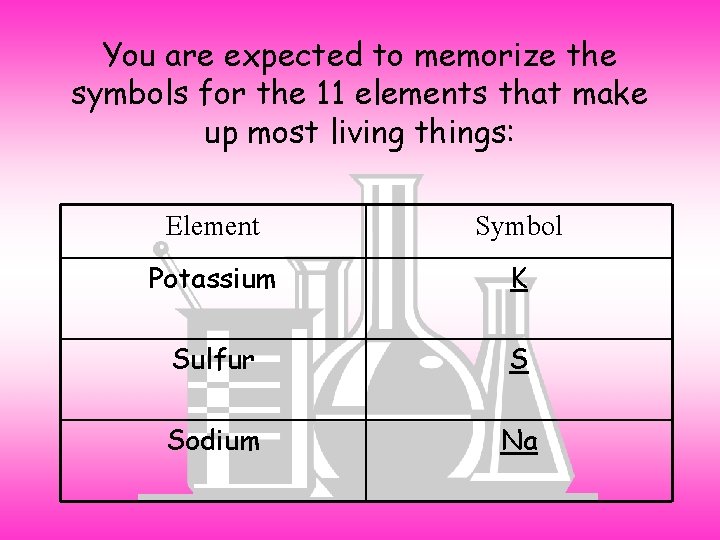
You are expected to memorize the symbols for the 11 elements that make up most living things: Element Symbol Potassium K Sulfur S Sodium Na

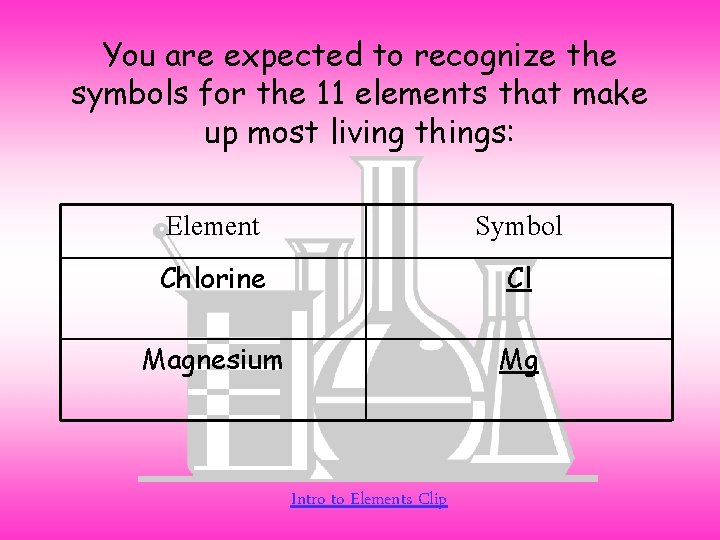
You are expected to recognize the symbols for the 11 elements that make up most living things: Element Symbol Chlorine Cl Magnesium Mg Intro to Elements Clip

Atoms combine together to form most of the substances we observe in the world around us. Compounds are formed when the atoms of two or more elements chemically joined together to form a new substance with very different properties than the elements of which it is formed.

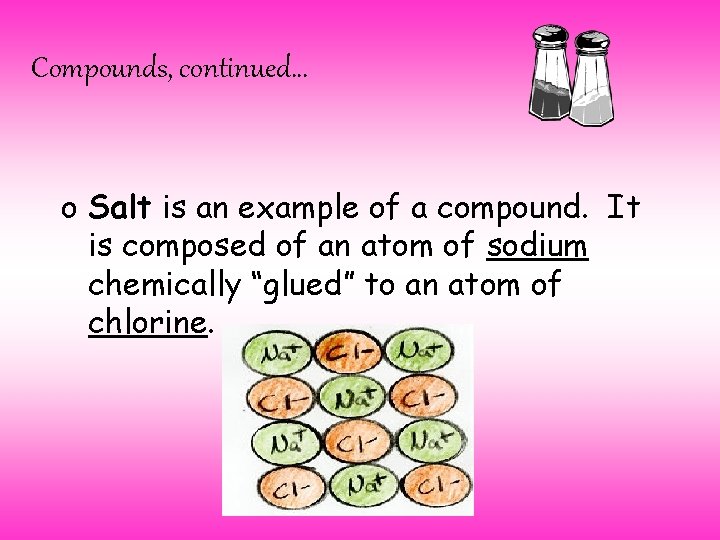
Compounds, continued… o Salt is an example of a compound. It is composed of an atom of sodium chemically “glued” to an atom of chlorine.

Any Questions so far on Guided notes handout? ? ?

Mixtures are formed when two or more substances are mixed together but not chemically combined. Each substance retains its own characteristics, and can be separated from each other by simple physical means. The air in this room is a mixture of nitrogen, oxygen and small amounts of other gases. Compounds & Mixtures Clip

Reading Chemical Formulas: H 2 O ØThe letters tell you what elements make up the molecule…what are the elements in this molecule? ØThe little numbers to the right of the letters tell you HOW MANY ATOMS of each element. If there isn’t one, it just means that there is one atom of that element. ØIf there was a number in front of the formula, it would tell you how many MOLECULES of that substance there was: 4 H 2 O How many molecules of water are there?

There are two types of compounds that are important to living things: Organic and inorganic compounds Organic compounds contain the element carbon. There are 4 types of organic compounds that are important to living things: (FILL IN YOUR CHARTS)

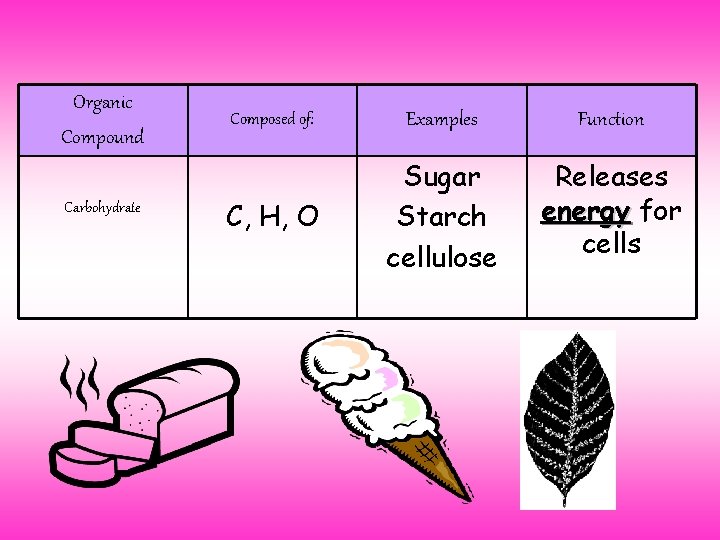
Organic Compound Carbohydrate Composed of: Examples Function C, H, O Sugar Starch cellulose Releases energy for cells

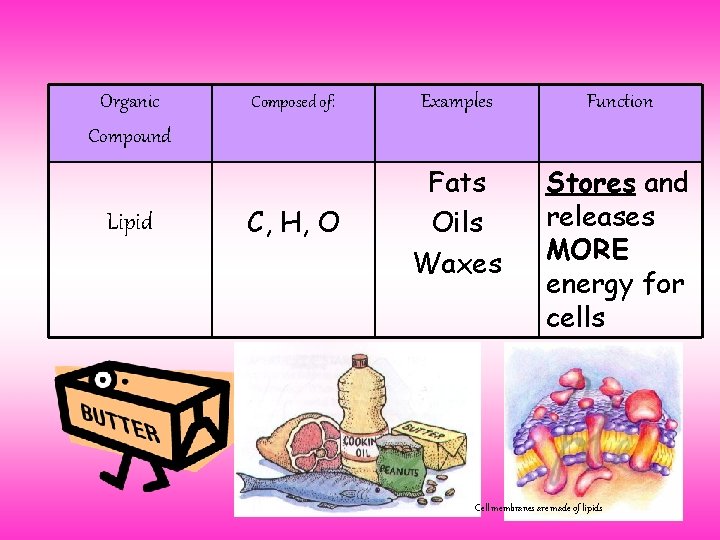
Organic Compound Lipid Composed of: Examples Function C, H, O Fats Oils Waxes Stores and releases MORE energy for cells Cell membranes are made of lipids

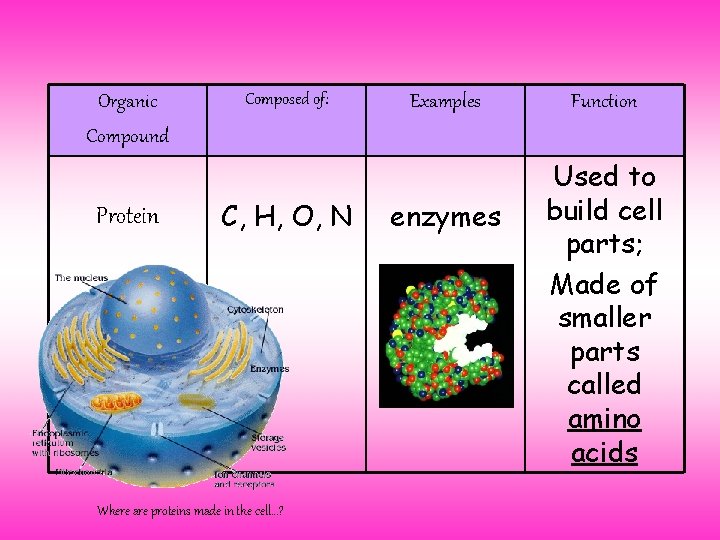
Organic Compound Protein Composed of: C, H, O, N Where are proteins made in the cell…? Examples enzymes Function Used to build cell parts; Made of smaller parts called amino acids

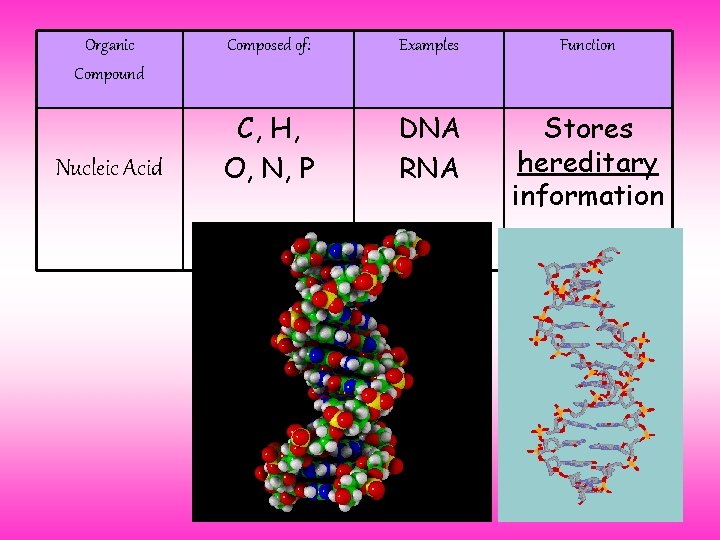
Organic Compound Nucleic Acid Composed of: Examples Function C, H, O, N, P DNA RNA Stores hereditary information

Inorganic compounds are also important to living things. They DO NOT contain carbon. Some examples include: • water (formula: H 2 O) • salt (formula: Na. Cl) Simple Chemistry Clip

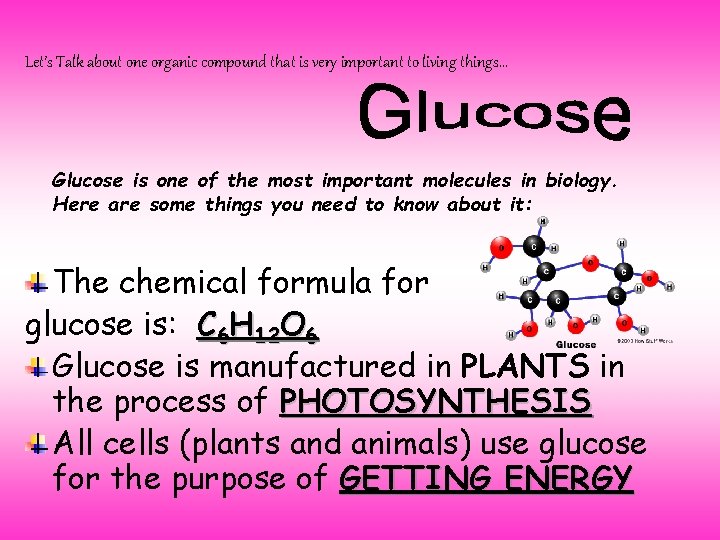
Let’s Talk about one organic compound that is very important to living things… Glucose is one of the most important molecules in biology. Here are some things you need to know about it: The chemical formula for glucose is: C 6 H 12 O 6 Glucose is manufactured in PLANTS in the process of PHOTOSYNTHESIS All cells (plants and animals) use glucose for the purpose of GETTING ENERGY

Let’s Talk about one organic compound that is very important to living things… • All energy in a glucose molecule is located in THE BONDS • When the bonds of this molecule are broken, energy is released. This happens in the MITOCHONDRIA of the cell (the powerhouse organelle).
 Anything that occupies space
Anything that occupies space Is anything that has mass and takes up space.
Is anything that has mass and takes up space. Anything that has matter and takes up space
Anything that has matter and takes up space Defintion of mass
Defintion of mass Mass
Mass Anything that has mass and take up space
Anything that has mass and take up space Anything that has mass and takes up space
Anything that has mass and takes up space Matter is anything that has both
Matter is anything that has both Is matter anything that has mass
Is matter anything that has mass Whats anything that has mass and takes up space
Whats anything that has mass and takes up space Anything that takes up space and has mass
Anything that takes up space and has mass What is anything that has mass and volume
What is anything that has mass and volume Matter is anything that occupies
Matter is anything that occupies Matter is anything that occupies space and has
Matter is anything that occupies space and has It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter has mass and occupies space
Matter has mass and occupies space Phân độ lown
Phân độ lown Block nhĩ thất độ 2 mobitz 2
Block nhĩ thất độ 2 mobitz 2 Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật


































