MATTER Matter anything that has mass takes up









- Slides: 18

MATTER

Matter anything that has mass & takes up space Mass the amount of matter an object contains

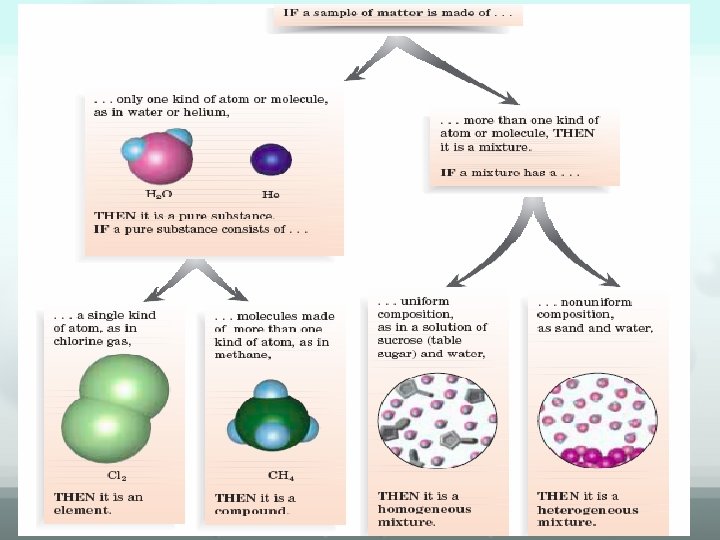
SUBSTANCES • Matter that has a uniform and definite composition is called a substance. (also known as pure substances) • Contain only one kind of matter • Which is not a pure substance? (sugar, water, lemonade)

PROPERTIES OF MATTER • Physical Properties: can be observed or measured without changing the composition of the substance (i. e. . State of matter, color, Tº, mass, density)

Intensive & Extensive Properties • Intensive – independent of the amount matter present. (density, m. p. , b. p. , color, ability to conduct electricity) • Extensive – does depend on the amount of matter in the substance. (volume, mass, amount of energy in a substance)

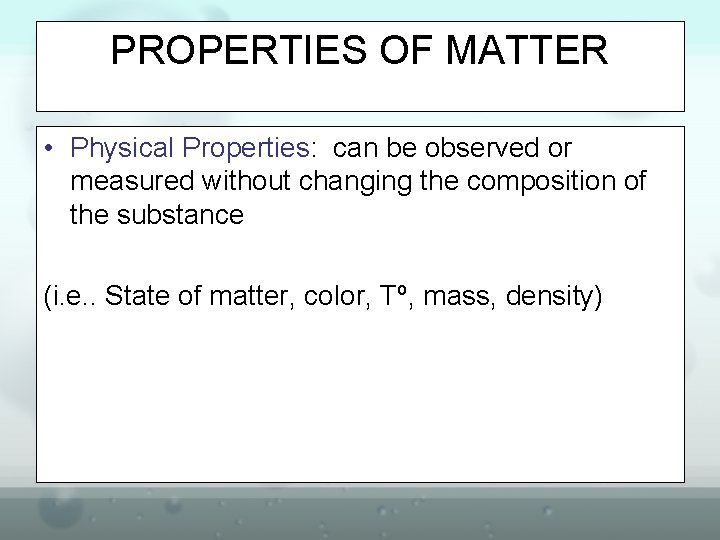
States of Matter • Solid – – definite shape – definite volume – Particles of a solid are packed close together and the motion seems to be a vibration.

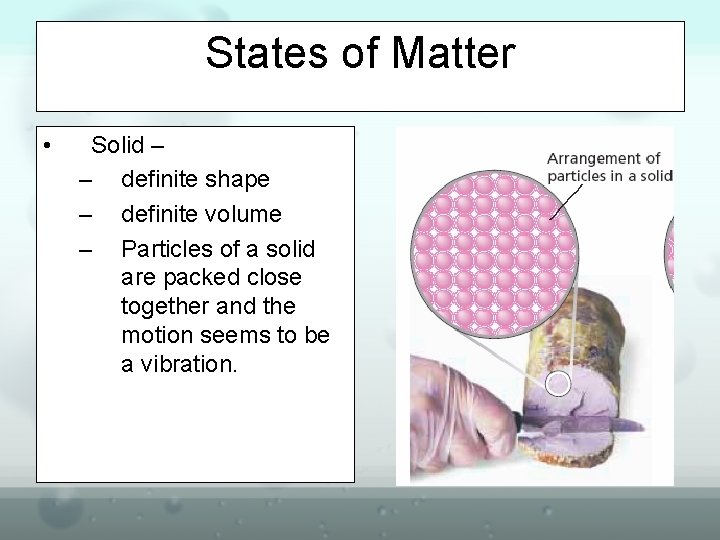
States of Matter • Liquid – – – • no definite shape definite volume Particles of a liquid bump into each other; they push the particles farther apart and move past them. This causes the "flow" of a liquid. Vapor – a substance in the gaseous state which is usually a solid or liquid at room temperature (water vapor)

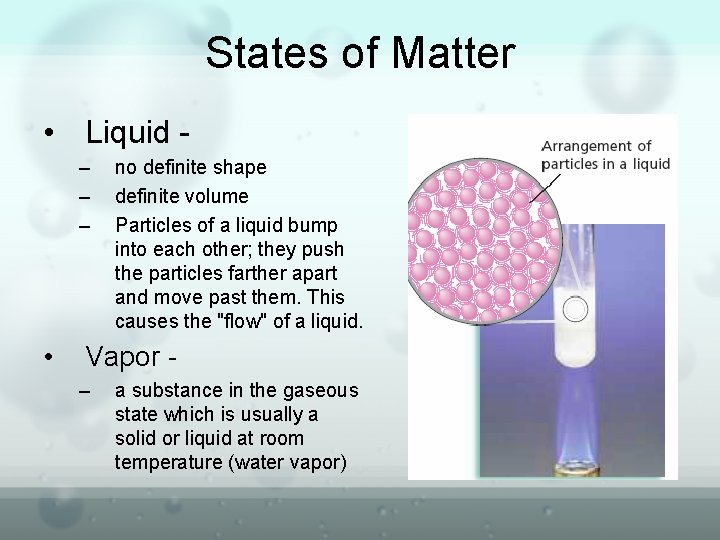
States of Matter • Gas – – no definite shape – no definite volume – The particles collide with enough force to push the particles so far apart that they escape the attraction of the surrounding particles. – high compressibility

Physical Changes • alters a substance without changing the composition. – Tearing paper, all change states: freezing, evaporation, condensation, deposition, also things like chromatography, distillation, etc.

Elements The simplest form of matter • An element cannot be changed into simpler substances by any chemical process. • Elements are made up of atoms • HOMOGENEOUS • Building blocks for all other substances ( what things ? )

Compounds Made up of two or more elements chemically combined • Can only be separated by chemical means • HOMOGENEOUS • Constituent elements lose their original properties • Sodium + chlorine sodium chloride • Always present in the same ratio in a compound

YIELDS ARROW • means changes or produces and is used to write chemical reactions

MIXTURES Two or more substances physically combined – can be separated by physical means – constituent substances retain their original properties – (salt & water - salt water)

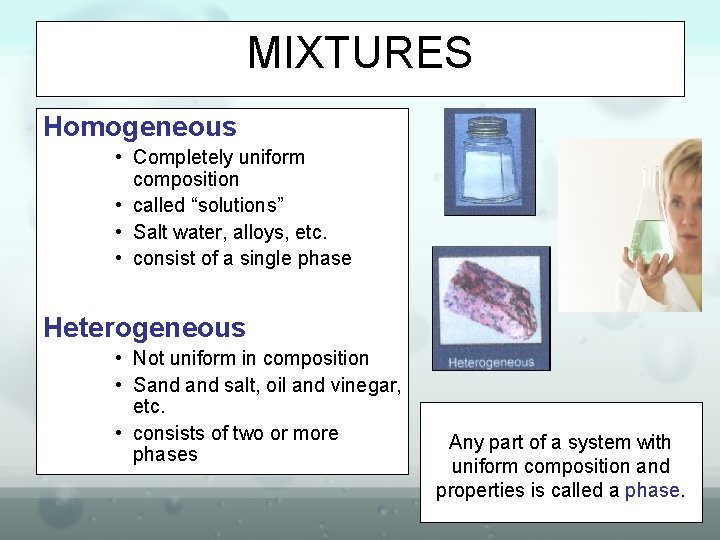
MIXTURES Homogeneous • Completely uniform composition • called “solutions” • Salt water, alloys, etc. • consist of a single phase Heterogeneous • Not uniform in composition • Sand salt, oil and vinegar, etc. • consists of two or more phases Any part of a system with uniform composition and properties is called a phase.

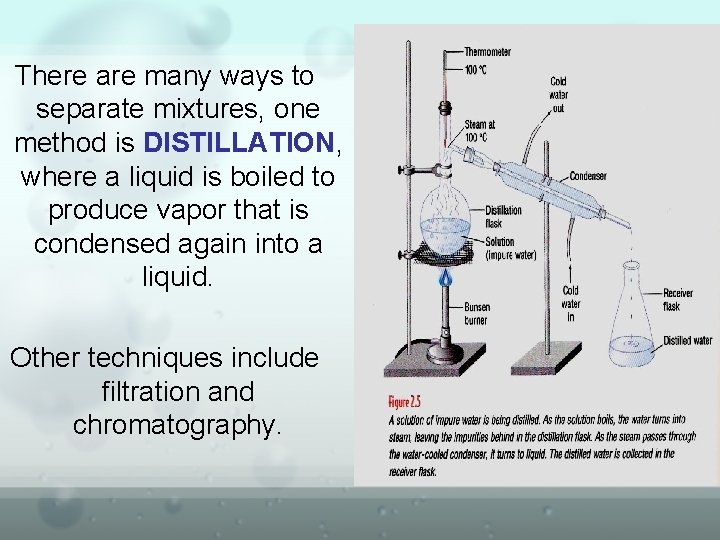
There are many ways to separate mixtures, one method is DISTILLATION, where a liquid is boiled to produce vapor that is condensed again into a liquid. Other techniques include filtration and chromatography.

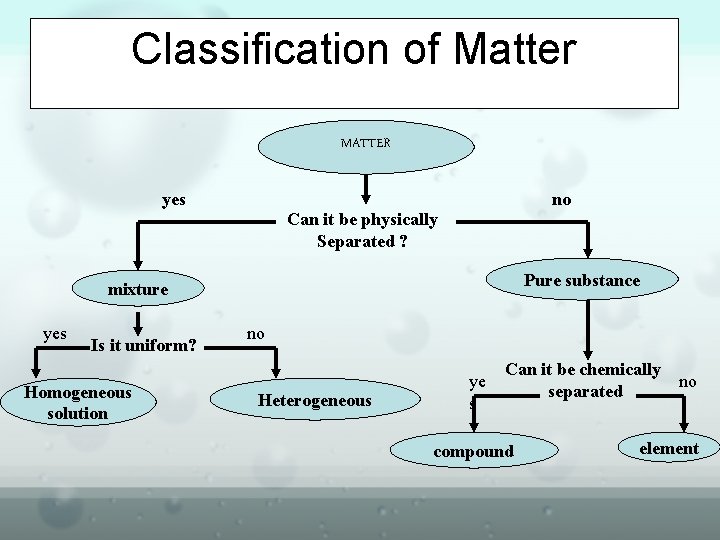
Classification of Matter MATTER yes no Can it be physically Separated ? Pure substance mixture yes Is it uniform? Homogeneous solution no Heterogeneous ye s Can it be chemically no separated compound element


Pure Substances and Mixtures