matter matter Anything that has mass and takes











































- Slides: 52

matter

matter • Anything that has mass and takes up space

chemistry

chemistry • Study of matter and how it changes

Physical property

Physical property • Characteristic that can be observed without changing it into a different substance • Example: freezing point, texture, color, temperature

Chemical property

Chemical property • Ability to change into difference substances • Example: rusting, flammability

Physical change

Physical change • Alters the form or appearance of matter but does not turn any substance into different substance • Examples: changes in states of matter (melting of snow), cutting, folding, shredding

Chemical change

Chemical change • Chemical reaction • Substance transforms into another substance • Example: burning wood ashes + gas

endothermic

Endothermic change • Change in which energy is absorbed Example: melting of ice *Feels cool

Exothermic change

Exothermic change • Energy is given off • Feels warm

mixture

mixture Two or more materials combined together Example: salad, hot chocolate, pancakes

solution

solution • Special mixture when a material dissolves in water Example: salt water

dissolving

dissolving • One material disperses evenly into another material so the first one seems to disappear Example: stirring sugar in tea

Post-it Check 1. Name the 3 mixtures you made. 2. How was the salt and water different from the others after you added water and stirred? 3. What are the two ways used for separating mixtures?

evaporation

evaporation • Liquid turns to gas and disperses to the air, leaving any dissolved solid material behind

crystal

crystal • Solid form of a material that can be identified by its properties such as shape, color, and pattern

Question? ? Your challenge is to design a method to separate this mixture of solid materials so that the gravel ends in the G cup, the powder in the P cup, and the salt in the S cup. Hmmm… Have you designed your method? Have you separated the gravel? Have you separated the powder? Have you separated the salt?

Question? ? How much salt can you dissolve in 50 ml of water? Materials: Water bottle post-it Funnel Salt Blue scoop Filter Balance Gram pieces Syringe Tub of water

Saturating a Solution 1. Add 50 ml to the bottle 2. Add one scoop of salt at a time, put the cap on and shake. Keep doing this until you see salt at the bottom. 3. Pour the bottle through the funnel using a wet coffee filter. What remains is the saturated solution. 4. Find the mass of your saturated solution using a balance. Put the saturated solution on one side and 50 ml of plain water on the other. Add gram pieces to the plain water until they are balanced.

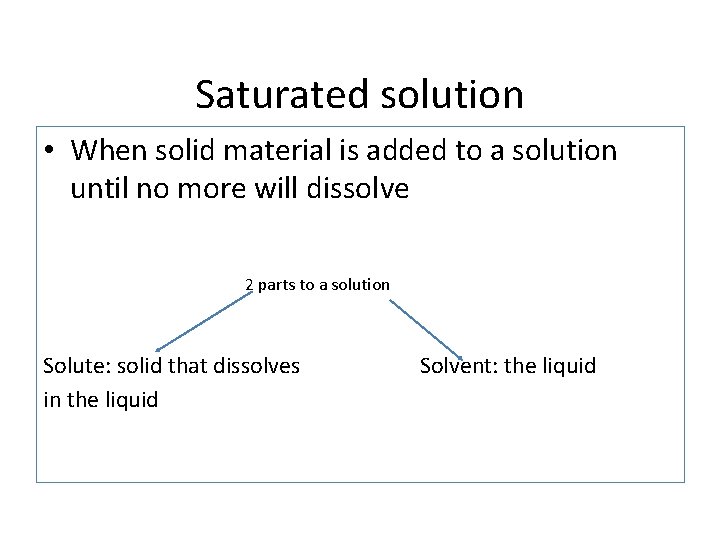
Saturated solution

Saturated solution • When solid material is added to a solution until no more will dissolve 2 parts to a solution Solute: solid that dissolves in the liquid Solvent: the liquid

Post-it Check Complete this Claims and Evidence Claims Evidence I know it took _____ grams of Salt to saturate 50 ml of water. I know this because…

Heterogeneous mixture

• Can see the different parts and can easily be separated • Example: salad

Homogeneous mixture

Homogeneous mixture • So evenly mixed can’t see the different parts • Example: solution, air

solubility

solubility • The property that substances have of dissolving in solvents

concentration

concentration • The amount of material dissolved in a volume (measurement) of liquid.

element

element • Substance that cannot be broken down into any other substances • Example: aluminum, hydrogen, carbon, calcium, nitrogen, silver, gold, mercury • They each have a one or two letter symbol • O=oxygen • C=carbon • Fe=iron

atom

atom • Basic particle from which all elements are made

molecule

molecule • A group of two or more atoms held together by chemical bonds

compound • A substance made of two or more elements • When they combine, they form compounds with properties different from the elements. • Example: C 12 H 22011=table sugar It has 12 atoms of carbon, 22 atoms of hydrogen, and 11 atoms of oxygen Carbon is black but sugar is not Oxygen is a gas but sugar is not

Potential energy

Potential energy • Energy of an object because of its position

Kinetic energy

Kinetic energy • Energy of an object in motion
 Matter is anything that has mass and occupies space
Matter is anything that has mass and occupies space What is mass
What is mass Anything that has mass
Anything that has mass Matter is anything that has and takes up
Matter is anything that has and takes up Anything that has mass and takes up space
Anything that has mass and takes up space Matter is anything that
Matter is anything that Matter is anything that has mass
Matter is anything that has mass Anything that has mass and volume is
Anything that has mass and volume is Anything that takes up space and has mass
Anything that takes up space and has mass Anything that has mass and takes up space
Anything that has mass and takes up space Anything that takes up space
Anything that takes up space Matter is anything with mass and volume
Matter is anything with mass and volume What is anything that occupies space and has mass?
What is anything that occupies space and has mass? It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter has mass and occupies space
Matter has mass and occupies space Matter is anything that:
Matter is anything that: Phân độ lown
Phân độ lown Block nhĩ thất độ 1
Block nhĩ thất độ 1 Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm vết của đường thẳng
Tìm vết của đường thẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Frank has an eraser
Frank has an eraser Matter is defined as anything that
Matter is defined as anything that Matter anything that
Matter anything that Use of density
Use of density Matter is anything that:
Matter is anything that: What is matter
What is matter No matter anything
No matter anything Matter is anything that
Matter is anything that No matter anything
No matter anything Matter anything that
Matter anything that Benjamin cummings
Benjamin cummings Matter anything that
Matter anything that Matter anything that
Matter anything that Everyone has what it takes
Everyone has what it takes Composed upon westminster bridge figures of speech
Composed upon westminster bridge figures of speech Relative atomic mass of beryllium
Relative atomic mass of beryllium Difference between atomic number and atomic mass
Difference between atomic number and atomic mass Abundance calculation chemistry
Abundance calculation chemistry Magnesium atomic number
Magnesium atomic number V=density/mass
V=density/mass Mass and matter
Mass and matter Whats gray matter
Whats gray matter Optic tract
Optic tract Gray matter and white matter
Gray matter and white matter What is gray matter
What is gray matter A hockey puck sliding on smooth ice at 4 m/s
A hockey puck sliding on smooth ice at 4 m/s Ideal stoichiometric calculations
Ideal stoichiometric calculations