MATTER ANYTHING THAT HAS MASS AND TAKES UP




































- Slides: 47

MATTER ANYTHING THAT HAS MASS AND TAKES UP SPACE!

PHYSICAL PROPERTIES • PHYSICAL PROPERTIES CAN BE OBSERVED OR MEASURED WITHOUT CHANGING THE SUBSTANCE • PHYSICAL PROPERTIES CAN HELP YOU IDENTIFY AN OBJECT

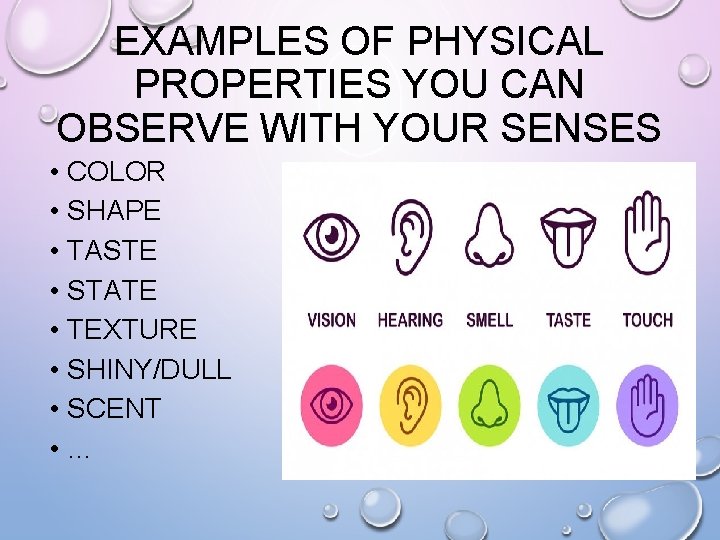
EXAMPLES OF PHYSICAL PROPERTIES YOU CAN OBSERVE WITH YOUR SENSES • COLOR • SHAPE • TASTE • STATE • TEXTURE • SHINY/DULL • SCENT • …

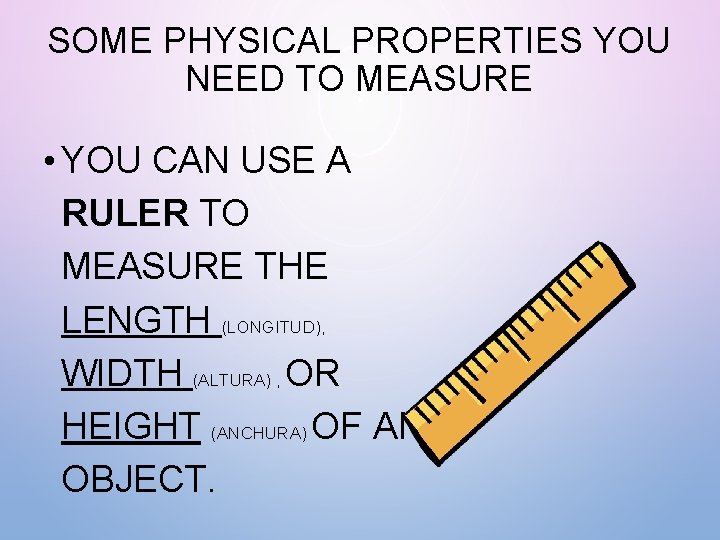
SOME PHYSICAL PROPERTIES YOU NEED TO MEASURE • YOU CAN USE A RULER TO MEASURE THE LENGTH (LONGITUD), WIDTH (ALTURA) , OR HEIGHT (ANCHURA) OF AN OBJECT.

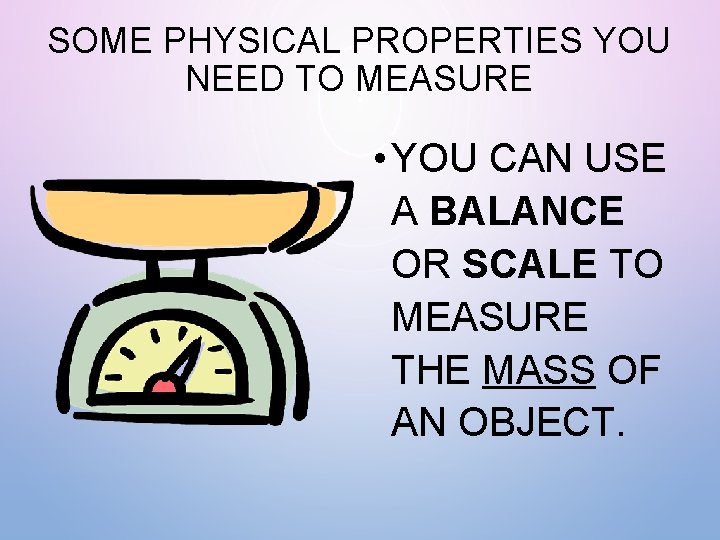
SOME PHYSICAL PROPERTIES YOU NEED TO MEASURE • YOU CAN USE A BALANCE OR SCALE TO MEASURE THE MASS OF AN OBJECT.

SOME PHYSICAL PROPERTIES YOU NEED TO MEASURE • YOU CAN USE A GRADUATED CYLINDER TO MEASURE THE VOLUME OF A LIQUID.

SOME PHYSICAL PROPERTIES YOU NEED TO MEASURE • YOU CAN PUT AN OBJECT IN WATER TO SEE IF IT DISSOLVES. • IF IT DOES, WE SAY THAT IT IS SOLUBLE IN WATER.

SOME PHYSICAL PROPERTIES YOU NEED TO MEASURE • MELTING AND BOILING POINTS ARE PHYSICAL PROPERTIES OF MATTER.

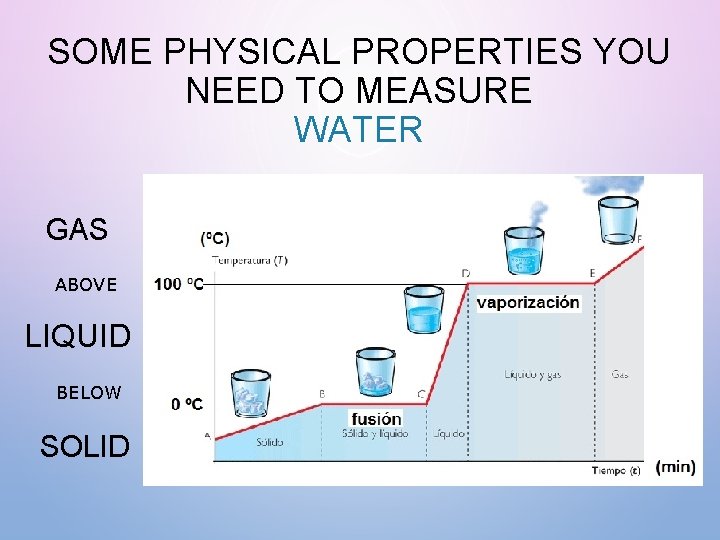
SOME PHYSICAL PROPERTIES YOU NEED TO MEASURE • MELTING POINT = THE TEMPERATURE AT WHICH A SOLID BECOMES A LIQUID. • WATER IS A LIQUID ABOVE 0 DEGREES CELSIUS AND BELOW 100 DEGREES CELSIUS.

SOME PHYSICAL PROPERTIES YOU NEED TO MEASURE WATER GAS ABOVE LIQUID BELOW SOLID

SOME PHYSICAL PROPERTIES YOU NEED TO MEASURE • BOILING POINT = THE TEMPERATURE AT WHICH A LIQUID CHANGES INTO A GAS • WATER IS A GAS (WATER VAPOR) ABOVE 100 DEGREES CELSIUS.

SOME PHYSICAL PROPERTIES YOU NEED TO MEASURE • WILL AN ITEM FLOAT IN WATER? • DENSITY AND BUOYANCY ARE PHYSICAL PROPERTIES!

HOW DO YOU CALCULATE DENSITY? • DENSITY = MASS DIVIDED BY VOLUME • THE DENSITY OF WATER IS 1 GRAM/CM 3 • IF THE DENSITY OF THE OBJECT IS LESS THAN 1 GRAM/CM 3, THE ITEM WILL FLOAT • IF THE DENSITY OF THE OBJECT IS GREATER THAN 1 GRAM/CM 3, THE ITEM WILL SINK

PHYSICAL CHANGES • A PHYSICAL CHANGE IS A CHANGE OF THE STATE, APPEARANCE, SHAPE, SIZE, OR TEXTURE OF A SUBSTANCE. • A PHYSICAL CHANGE DOES NOT CHANGE THE IDENTITY OF A SUBSTANCE.

EXAMPLES OF PHYSICAL CHANGES • CRUSHING AN POP CAN

EXAMPLES OF PHYSICAL CHANGES • TEARING OR CUTTING A PIECE OF PAPER

EXAMPLES OF PHYSICAL CHANGES • BREAKING A PENCIL

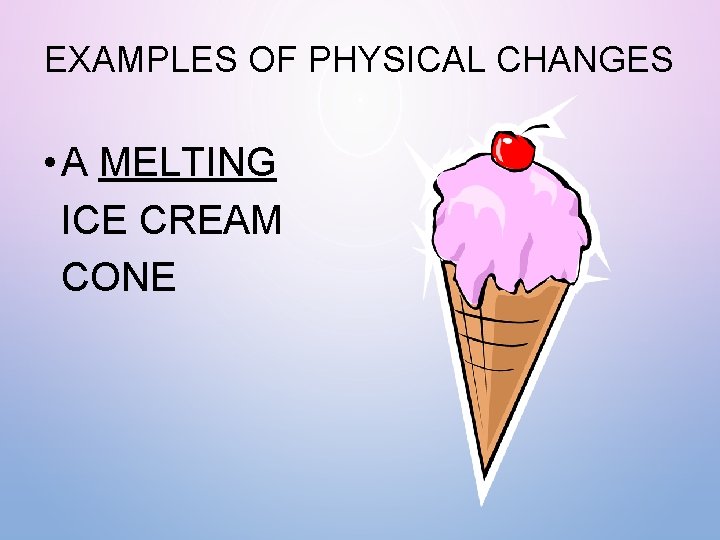
EXAMPLES OF PHYSICAL CHANGES • A MELTING ICE CREAM CONE

EXAMPLES OF PHYSICAL CHANGES • WATER EVAPORATING

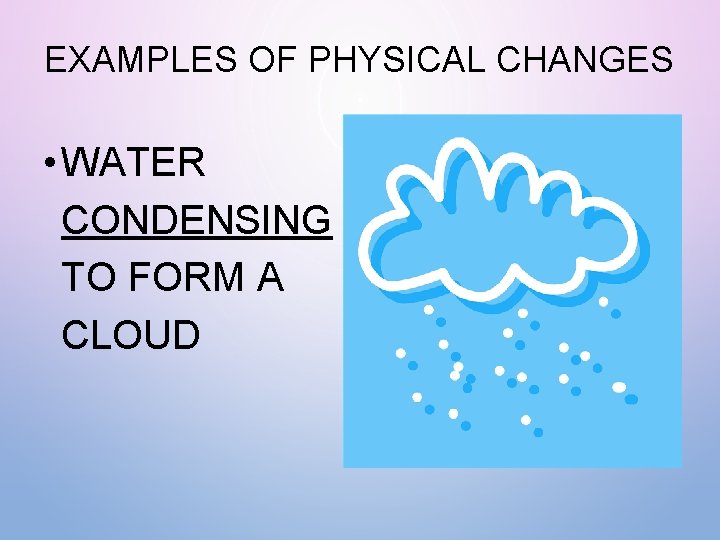
EXAMPLES OF PHYSICAL CHANGES • WATER CONDENSING TO FORM A CLOUD

CHEMICAL PROPERTIES • A CHEMICAL PROPERTY TELLS US HOW A SUBSTANCE WILL BEHAVE DURING A CHEMICAL REACTION. • CHEMICAL CHANGES HAPPEN DURING A CHEMICAL REACTION.

EXAMPLES OF CHEMICAL PROPERTIES • FLAMMABILITY = HOW EASILY A SUBSTANCE WILL CATCH ON FIRE

EXAMPLES OF CHEMICAL PROPERTIES • WILL AN ITEM RUST? • SOME MATERIALS RUST (IRON) • OTHER MATERIALS DO NOT RUST (ALUMINUM)

CHEMICAL CHANGES • A CHEMICAL CHANGE RESULTS IN THE FORMATION OF ONE OR MORE NEW SUBSTANCES • CHEMICAL CHANGES OCCUR ALL AROUND US! • CHEMICAL CHANGES ARE HAPPENING IN YOUR BODY RIGHT NOW!

CHEMICAL CHANGES ARE DUE TO CHEMICAL REACTIONS

WHAT HAPPENS DURING A CHEMICAL REACTION? • CHEMICAL REACTION = THE PROCESS BY WHICH NEW SUBSTANCES ARE FORMED • CHEMICAL REACTIONS ARE IRREVERSIBLE

WHAT HAPPENS DURING A CHEMICAL REACTION? • DURING A CHEMICAL REACTION, THE ATOMS ARE REARRANGED AND A NEW SUBSTANCE IS FORMED • THIS NEW SUBSTANCE OFTEN HAS DIFFERENT PHYSICAL AND CHEMICAL PROPERTIES COMPARED TO THE ORIGINAL SUBSTANCE

EXAMPLES OF CHEMICAL REACTIONS THAT CAUSE CHEMICAL CHANGES • DIGESTION = STOMACH ACIDS DIGEST YOUR FOOD

EXAMPLES OF CHEMICAL REACTIONS THAT CAUSE CHEMICAL CHANGES • CELLULAR RESPIRATION = CELLS RELEASE THE ENERGY STORED IN FOOD

EXAMPLES OF CHEMICAL REACTIONS THAT CAUSE CHEMICAL CHANGES • COOKING = USES HEAT TO CHANGE SUBSTANCES (EGGS, SUGAR, BUTTER, ETC. ) INTO NEW SUBSTANCES (COOKIES)

EXAMPLES OF CHEMICAL REACTIONS THAT CAUSE CHEMICAL CHANGES • BURNING = WOOD IS CHANGED INTO ASH, SMOKE, HEAT, AND LIGHT

EXAMPLES OF CHEMICAL REACTIONS THAT CAUSE CHEMICAL CHANGES • RUSTING = OXYGEN REACTS WITH SOME METALS AND CHANGES THEM INTO NEW MATERIALS

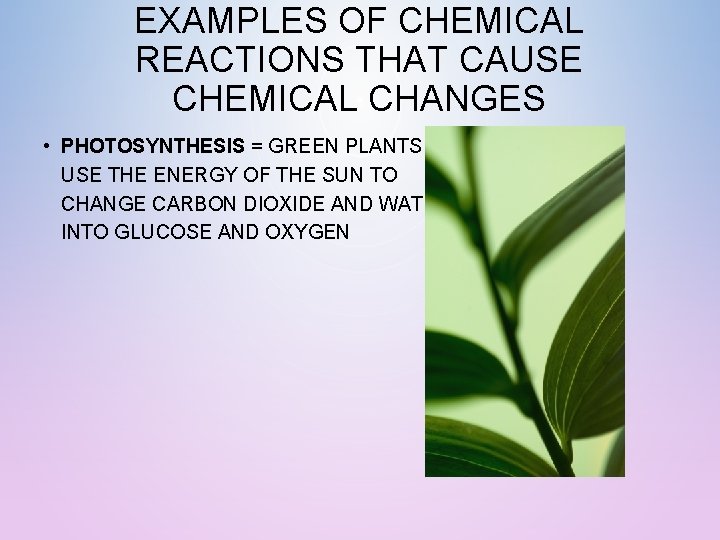
EXAMPLES OF CHEMICAL REACTIONS THAT CAUSE CHEMICAL CHANGES • PHOTOSYNTHESIS = GREEN PLANTS USE THE ENERGY OF THE SUN TO CHANGE CARBON DIOXIDE AND WATER INTO GLUCOSE AND OXYGEN

SLIDESHARE HTTPS: //SLIDEPLAYE R. COM/SLIDE/634276 1/

OTHER COMMON CHEMICAL REACTIONS • HYDROGEN + OXYGEN = WATER • IRON + OXYGEN = RUST • SODIUM + CHLORINE = TABLE SALT • CELLULAR RESPIRATION • PHOTOSYNTHESIS • BAKING COOKIES

HOW DO YOU KNOW WHEN A CHEMICAL CHANGE HAS OCCURRED? • SIGNS THAT A CHEMICAL CHANGE HAS OCCURRED INCLUDE: • CHANGE IN TEMPERATURE • THE RELEASE OF A GAS • FIRE &/OR SMOKE • CHANGE IN COLOR • LIGHT IS PRODUCED

• WHAT HAPPENS TO THE AMOUNT OF MATTER DURING A CHEMICAL CHANGE? • IS THE AMOUNT OF MATTER INCREASED? • IS THE AMOUNT OF MATTER DECREASED?

DURING A CHEMICAL CHANGE, THERE IS NO INCREASE OR DECREASE IN THE QUANTITY OF MATTER. IN OTHER WORDS, THE NUMBER OF ATOMS DOES NOT INCREASE OR DECREASE; THEY JUST GET REARRANGED.

LAW OF CONSERVATION OF MATTER • MATTER CANNOT BE CREATED OR DESTROYED, BUT IT CAN CHANGE FORM. • THE TOTAL AMOUNT OF MATTER AND ENERGY AVAILABLE IN THE UNIVERSE IS A FIXED AMOUNT. • THERE IS NEVER ANY MORE OR LESS

LAW OF CONSERVATION OF MATTER • THE NUMBER OF ATOMS ON EACH SIDE OF A CHEMICAL EQUATION NEEDS TO BE THE SAME. • THIS IS CALLED BALANCING EQUATIONS. • WE BALANCE EQUATIONS BECAUSE OF THE LAW OF CONSERVATION OF MATTER

REVIEW • MATTER = ANYTHING THAT HAS MASS AND TAKES UP SPACE.

REVIEW • PHYSICAL PROPERTIES OF MATTER CAN BE DESCRIBED BY USING OUR SENSES (COLOR, SHAPE, SCENT, ETC. ) • PHYSICAL PROPERTIES OF MATTER CAN BE MEASURED (MASS, VOLUME, DENSITY, MELTING AND BOILING POINT,

REVIEW • MATTER CAN BE DESCRIBED BY HOW IT WILL BEHAVE DURING A CHEMICAL REACTION • SOME CHEMICAL PROPERTIES INCLUDE FLAMMABILITY AND THE ABILITY OF A SUBSTANCE TO RUST

REVIEW • CHANGES TO MATTER CAN BE PHYSICAL OR CHEMICAL. • SOME CHANGES DO NOT CHANGE THE MAKEUP OF THE MATTER (PHYSICAL CHANGES) • EXAMPLES OF PHYSICAL CHANGES INCLUDE: CUTTING, FREEZING, CRUSHING, BREAKING, MELTING,

REVIEW • SOME CHANGES TURN THE MATTER INTO ENTIRELY DIFFERENT SUBSTANCES (CHEMICAL CHANGES) • EXAMPLES OF CHEMICAL CHANGES INCLUDE: BURNING, RUSTING, COOKING, DIGESTING, PHOTOSYNTHESIS, CELLULAR RESPIRATION, ETC.

REVIEW • SIGNS THAT A CHEMICAL CHANGE HAS OCCURRED INCLUDE: • CHANGE IN TEMPERATURE • THE RELEASE OF A GAS • FIRE &/OR SMOKE • CHANGE IN COLOR • LIGHT IS PRODUCED

REVIEW • THE LAW OF CONSERVATION OF MATTER STATES THAT MATTER CANNOT BE CREATED OR DESTROYED, BUT IT CAN CHANGE FORM.