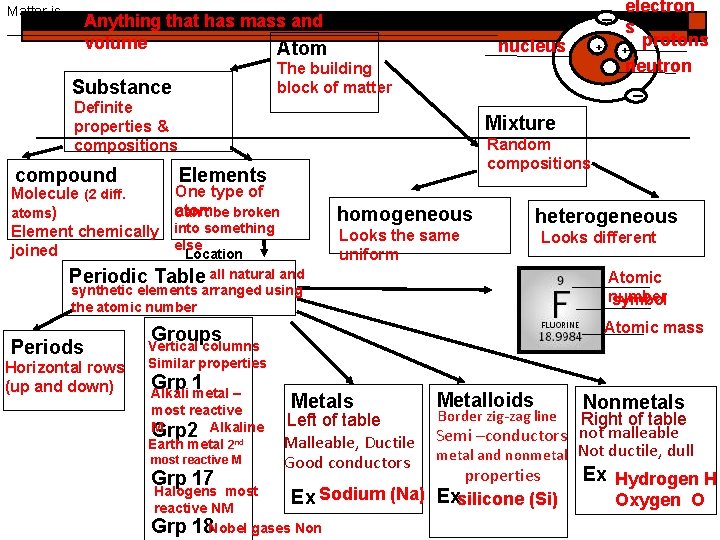
Matter is Anything that has mass and volume






















- Slides: 46

Matter is Anything that has mass and ________________________________ volume Atom Definite properties & compositions Molecule (2 diff. atoms) Element chemically joined Mixture Random compositions Elements One type of atombe broken Can’t into something else Location Periodic Table all natural and synthetic elements arranged using the atomic number Periods Horizontal rows (up and down) nucleus The building block of matter Substance compound _ electron s protons + + neutron _ homogeneous Looks the same uniform heterogeneous Looks different Atomic number symbol Atomic mass Groups Vertical columns Similar properties Grp 1 Alkali metal – most reactive M Grp 2 Alkaline Earth metal 2 nd most reactive M Grp 17 Metals Metalloids Border zig-zag line Nonmetals Right of table not malleable Semi –conductors metal and nonmetal Not ductile, dull properties Ex Hydrogen H Ex Sodium (Na) Exsilicone (Si) Oxygen O Left of table Malleable, Ductile Good conductors Halogens most reactive NM Grp 18 Nobel gases Non

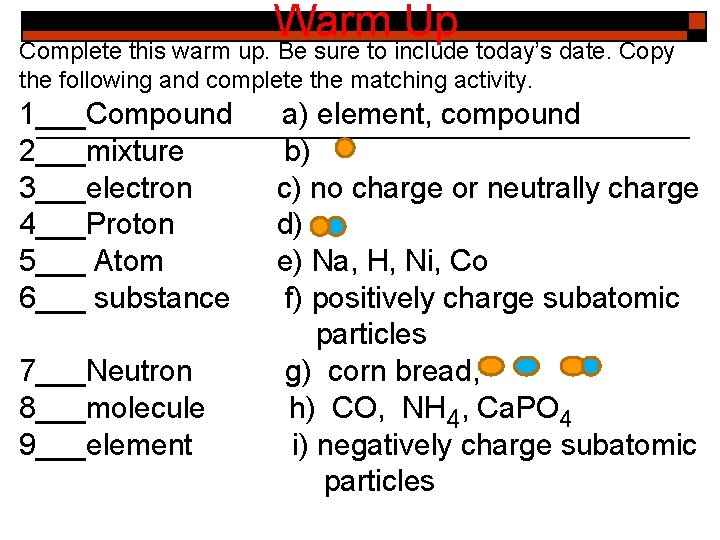
Warm Up Complete this warm up. Be sure to include today’s date. Copy the following and complete the matching activity. 1___Compound 2___mixture 3___electron 4___Proton 5___ Atom 6___ substance 7___Neutron 8___molecule 9___element a) element, compound b) c) no charge or neutrally charge d) e) Na, H, Ni, Co f) positively charge subatomic particles g) corn bread, h) CO, NH 4, Ca. PO 4 i) negatively charge subatomic particles

Physical and Chemical Changes magnetism

Everything that has mass and volume is called matter. Everything is made of matter

Matter has properties o Two basic types of properties that we can associate with matter. n Physical properties n Chemical properties

h o o What are properties? Characteristics used to identify, describe and classify matter Two basic types of properties of matter are : Physical properties and Chemical properties:

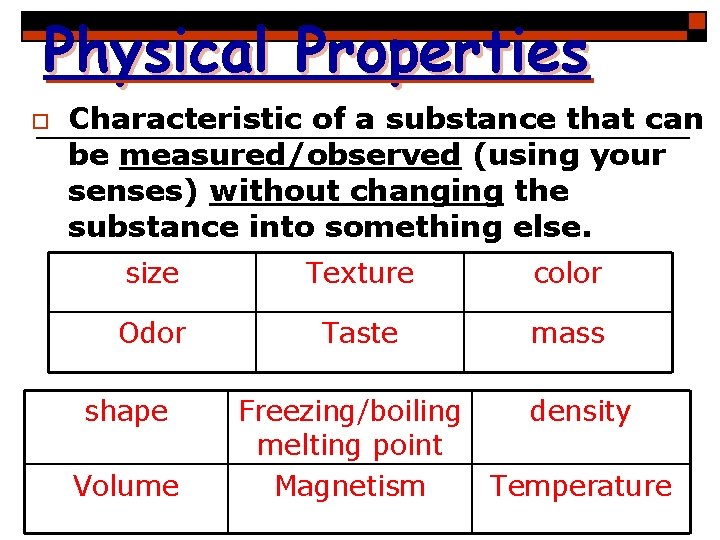
Physical Properties o Characteristic of a substance that can be measured/observed (using your senses) without changing the substance into something else. size Texture color Odor Taste mass shape Volume Freezing/boiling melting point Magnetism density Temperature

More EXAMPLES - Physical o Physical State solid, liquid, gas or plasma Viscosity - The resistance of a liquid to flowing. n n n Examples: Low viscosity-water, rubbing alcohol High viscosity-honey

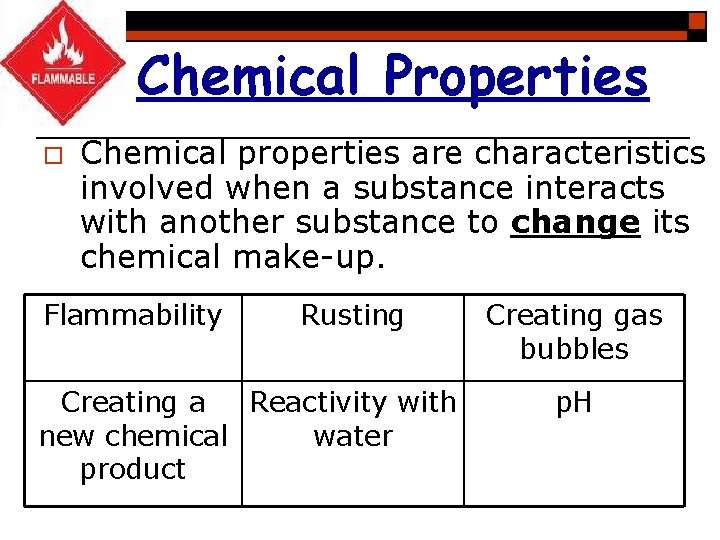
Chemical Properties o Chemical properties are characteristics involved when a substance interacts with another substance to change its chemical make-up. Flammability Rusting Creating a Reactivity with new chemical water product Creating gas bubbles p. H

Alike? Different? o Draw a double bubble map in your notes to compare and contrast physical and chemical properties.


Matter is everywhere. o Matter is anything that takes up space and has mass. o Matter is constantly experiencing both chemical and physical changes. o

Physical Change o o Physical changes occur when matter changes its physical property but not its chemical properties. No new substance is created. Physical property changes could include a change in: texture, shape, size, color, volume, mass, weight, and density.

Examples of Physical Changes o o o Boiling Freezing Dissolving Breaking Making a mixture n n n 2 or more types of matter (substances) mixed together Not in specific amounts Can be separated physically

Chemical Change Chemical changes are changes matter undergoes when it becomes new or different matter. o To identify a chemical change look for signs such as, bubbling and fizzing, light production, color change with an odor, smoke, and presence of heat. o

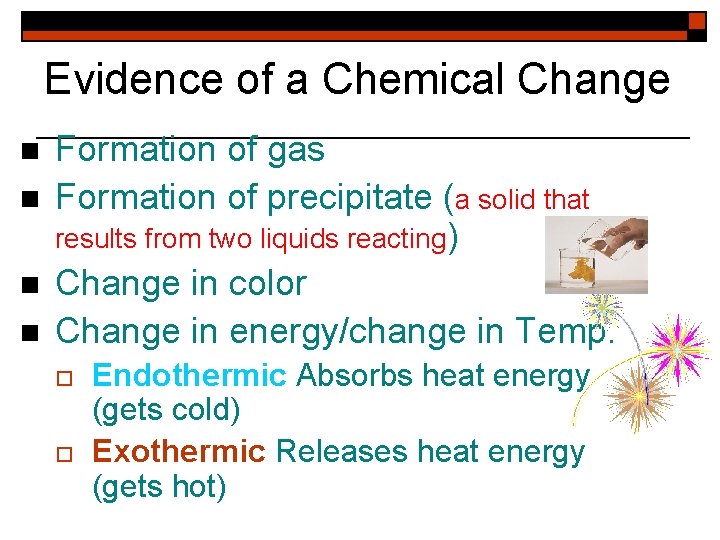
Evidence of a Chemical Change n n Formation of gas Formation of precipitate (a solid that results from two liquids reacting) Change in color Change in energy/change in Temp. o o Endothermic Absorbs heat energy (gets cold) Exothermic Releases heat energy (gets hot)

What kind of changes does matter undergo? The type of change that occurs in matter depends on the type of properties that are changed. Physical changes change physical properties only Chemical changes change chemical properties and physical properties

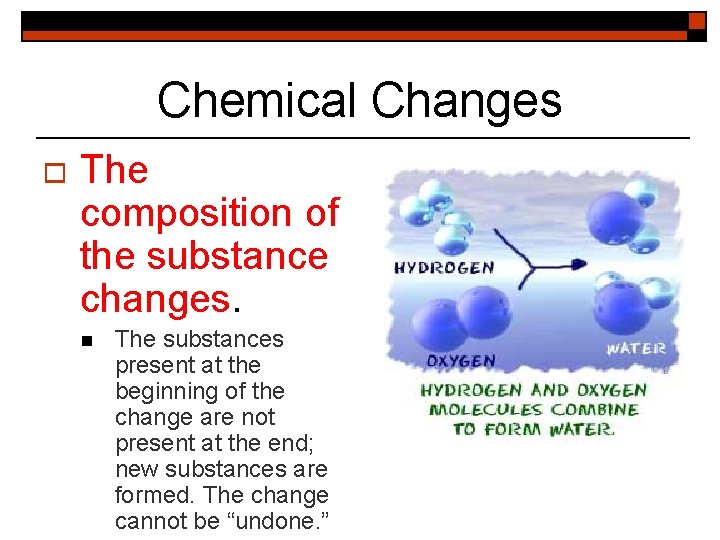
Chemical Changes o The composition of the substance changes. n The substances present at the beginning of the change are not present at the end; new substances are formed. The change cannot be “undone. ”

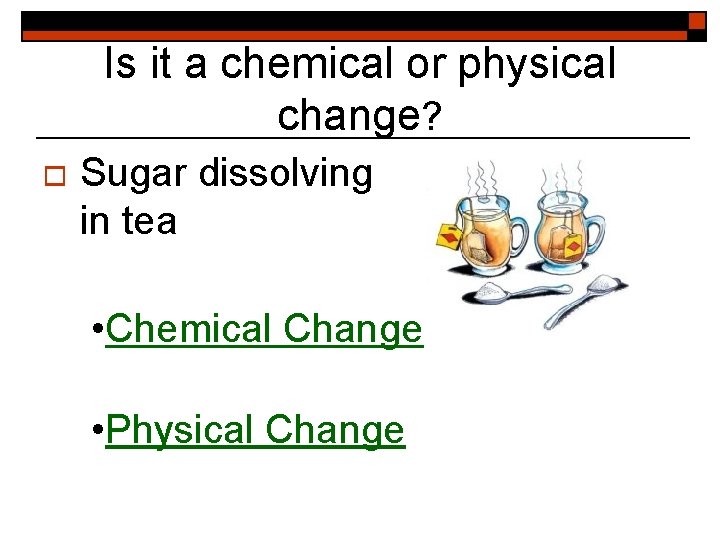
Is it a chemical or physical change? o Sugar dissolving in tea • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o Logs burning • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o Breaking water up by separating it into hydrogen and oxygen • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o Cutting paper • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o Crushing an aspirin • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o Metal rusting • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o Lighter fluid burning • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o An egg rotting • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Is it a chemical or physical change? o An egg breaking • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Writing Activity o Write a paragraph about the difference between a chemical and physical change. Give examples of each.