Flashcards for Unit 1 Matter Anything that has











































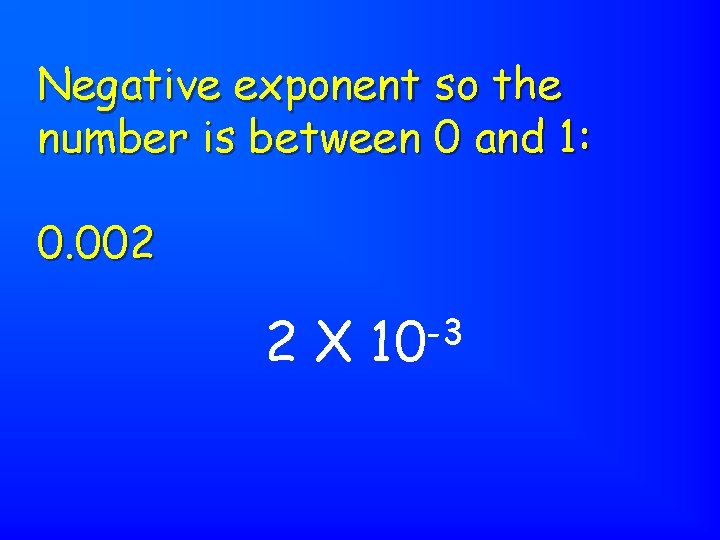













- Slides: 69

Flashcards for Unit 1

Matter Anything that has mass & occupies space.

A measure of the gravitational pull on matter. You would weigh less on the moon! Weight

A measure of the quantity of matter. You would have the same mass on the moon. Mass

A series of steps followed to solve problems, including collecting data, forming a hypothesis, testing the hypothesis, and stating conclusions. Scientific Method

A fact you take in with your senses. Observation

A testable statement Hypothesis

An inference Conclusion

Describes what happens. Often stated mathematically. Summarizes many (thousands of) observations. Scientific Law

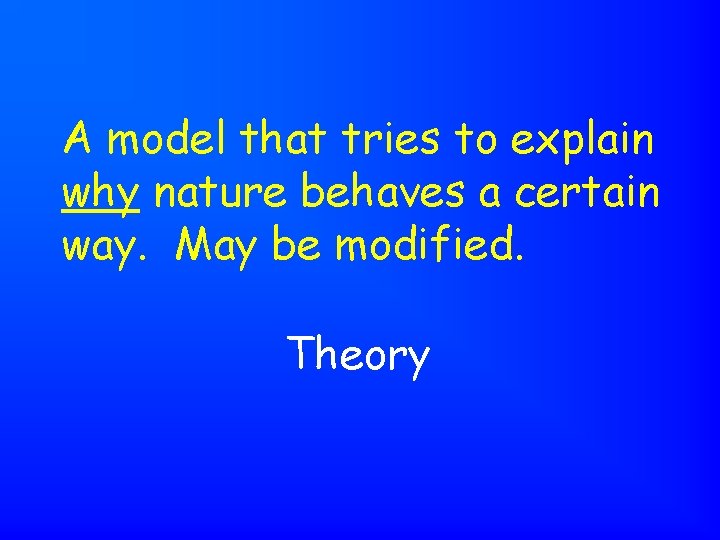
A model that tries to explain why nature behaves a certain way. May be modified. Theory

7 How many fundamental units in the SI system?

Fundamental or Base Unit Physical quantity that must be measured. It cannot be calculated.

Derived Unit A unit defined as a combination of fundamental units.

Unit of mass Kilogram Fundamental Unit

Unit of time Second Fundamental Unit

Unit of length Meter Fundamental Unit

Unit of temperature Kelvin Fundamental Unit

Space or Capacity Volume Derived Unit

Metric Unit of Volume 10 cm X 10 cm Liter Derived Unit

1000 ? 3 cm = 1 Liter Derived Unit

1000 m. L = 1 Liter ? milliliter = 1 Liter Derived Unit

1 m. L = 1 3 cm ? milliliter = 1 3 cm Derived Unit

1 kilogram What is the mass of 1 liter of pure H 2 O? Mass/Volume relationship

1 liter What is the volume of 1 kg of pure H 2 O? Mass/Volume relationship

1 gram What is the mass of 1 3 cm of pure H 2 O? Mass/Volume relationship

1 3 cm or 1 m. L What is the volume of 1 gram of pure H 2 O? Mass/Volume relationship

Unit of Mass Kilogram Unit of Time Second Unit of Length Meter Unit of Temperature Kelvin Unit of Amount of Substance Mole Unit of Volume: derived (Space or capacity) Liter, milliliter, cubic centimeter

273 K Freezing Point of water in the Kelvin scale. Physical Constant

373 K Boiling Point of water in the Kelvin scale. Physical Constant

0 C Freezing Point of water in the centigrade scale. Physical Constant

100 C Boiling Point of water in the centigrade scale. Physical Constant

Another name for freezing point. Ice / water equilibrium

Another name for boiling point. Steam / water equilibrium

100 cm = 1 meter ? cm in 1 meter Conversion Fact

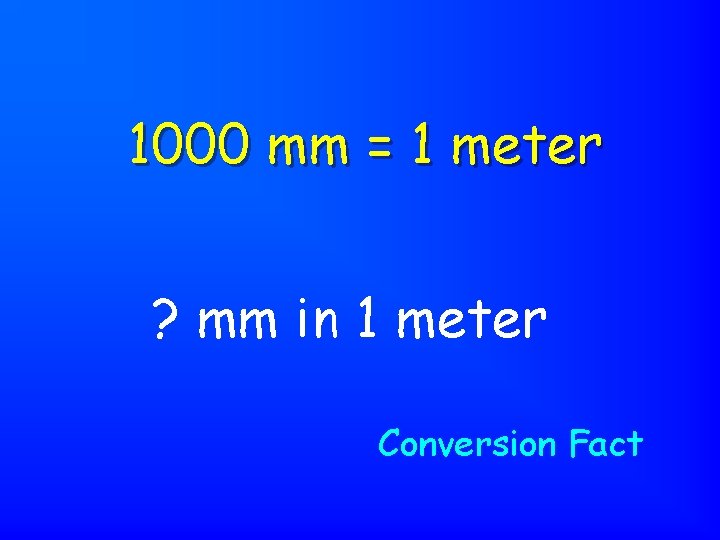
1000 mm = 1 meter ? mm in 1 meter Conversion Fact

1000 m = 1 kilometer ? m in 1 km Conversion Fact

1000 mg = 1 gram ? mg in 1 g Conversion Fact

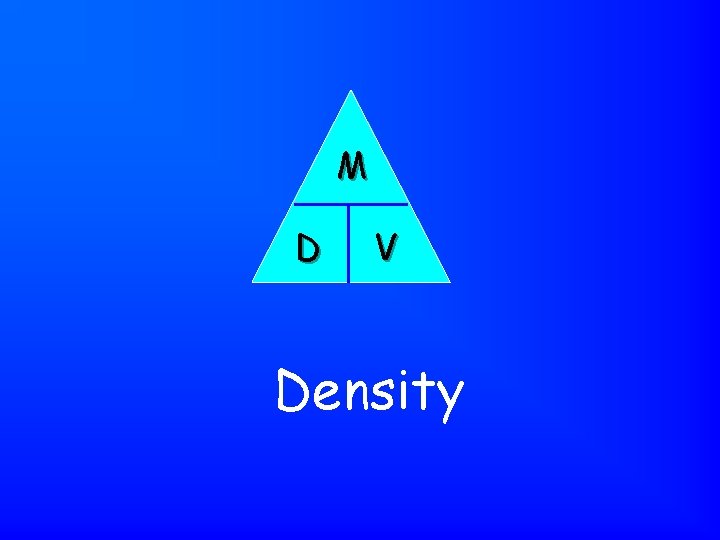
Mass / Volume Density

Describes how matter is packed into space. Density

grams / cm 3 for solids grams / ml for liquids Units of Density

M D V Density

How close a measured value is to an accepted value. Accuracy

How close a series of measurements are to one another. Precision

Low Scatter High Precision

Measured value – Accepted value x 100% Accepted value Percent Error Table T, reference tables

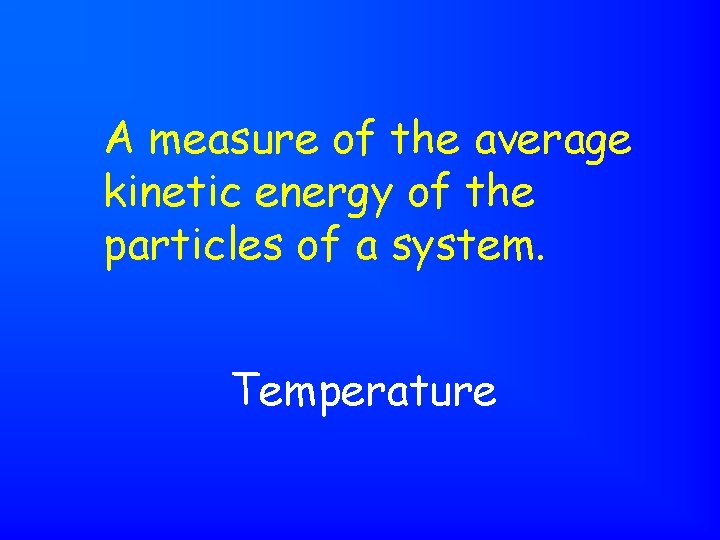
A measure of the average kinetic energy of the particles of a system. Temperature

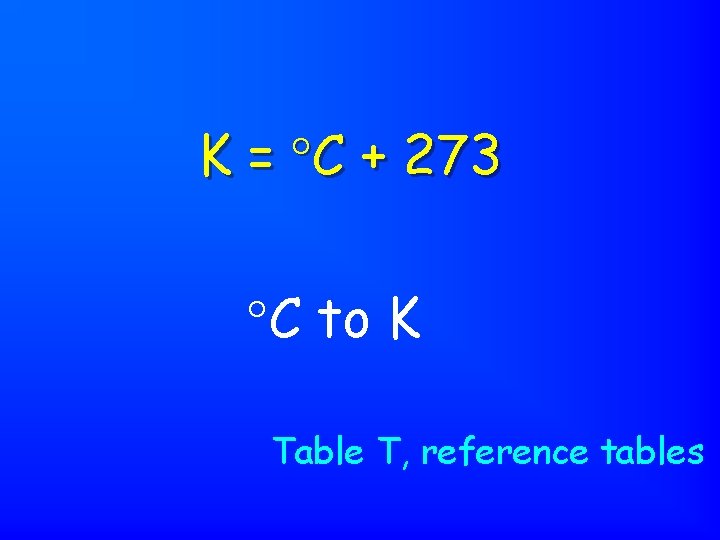
K = C + 273 C to K Table T, reference tables

The number is written as a product of 2 numbers: - a number between 1 & 10 - a power of 10 Scientific Notation
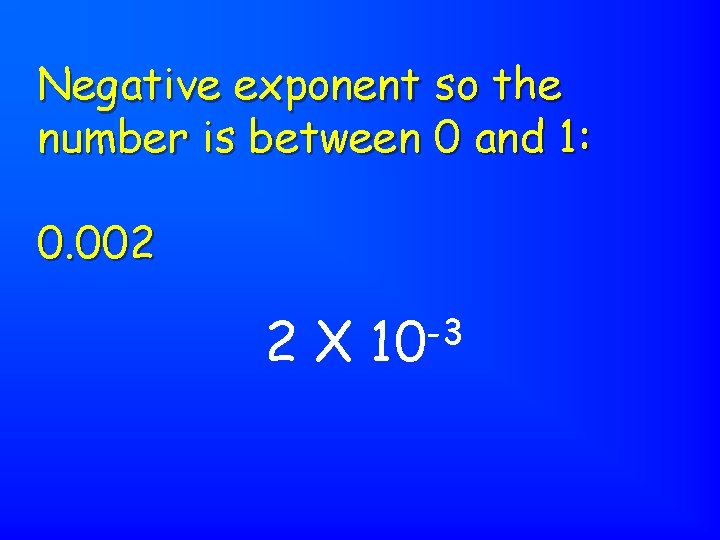
Negative exponent so the number is between 0 and 1: 0. 002 2 X -3 10

Positive exponent so the number is greater than 1: 500 5 X 2 10

3. 45 X The 1 st factor got smaller by a factor of 100. -17 10 So the 2 nd factor must increase by a factor of 100. Convert 345 X 10 -19 to scientific notation.

All known digits plus 1 estimated digit. Significant Figures From the perspective of the person MAKING the measurement.

Decimal Present – Pacific side Start counting at 1 st nonzero # and count until the end of the number. 5400. 145 cm 1 2 34 567 Interpreting someone else’s measurements. 7 sig figs

Decimal Present – Pacific side Start counting at 1 st nonzero # and count until the end of the number. 0. 0175 g 12 3 Interpreting someone else’s measurements. 3 sig figs

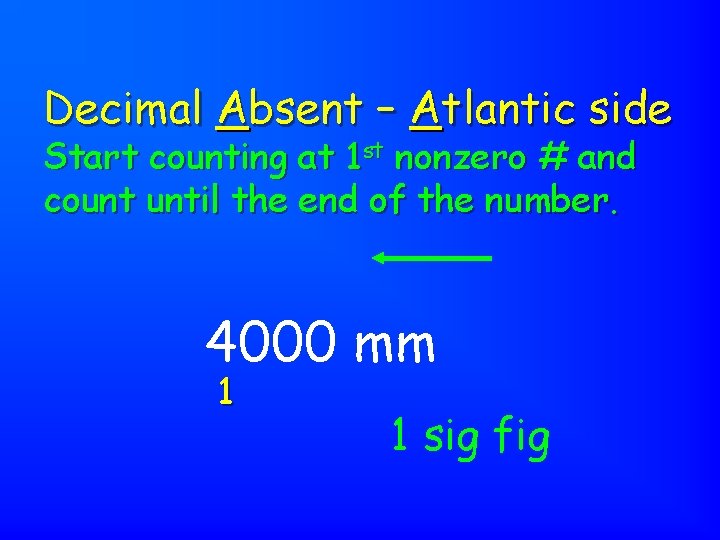
Decimal Absent – Atlantic side Start counting at 1 st nonzero # and count until the end of the number. 4855 g 4 321 4 sig figs

Decimal Absent – Atlantic side Start counting at 1 st nonzero # and count until the end of the number. 4000 mm 1 1 sig fig

Answer has same number of decimal places as the addend with the least number of decimal places. Rule for sig figs in Addition and Subtraction

77. 2 cm is correct! Least # of decimal places ! 28. 0 cm 23. 538 cm + 25. 68 cm 77. 218 cm But what do you report?

Answer has same number of significant figures as the factor with the least number of significant figures. Rule for sig figs in Multiplication & Division

78 m 2 is correct! 2 sig figs 3 sig figs 24 m X 3. 26 m = 78. 24 But what do you report? 2 m

a) 2. 749 g/cm 3 b) 2. 75 g/cm 3 c) 2. 7 g/cm 3 d) 0. 36 cm 3/g 3 sig figs An aluminum cube has a mass of 4. 75 grams. The dimensions of the cube are 1. 2 cm X 1. 2 cm. What is the density of the 2 sf cube? “Volume” = 1. 728 cm 3 “Volume” = 1. 728 cm

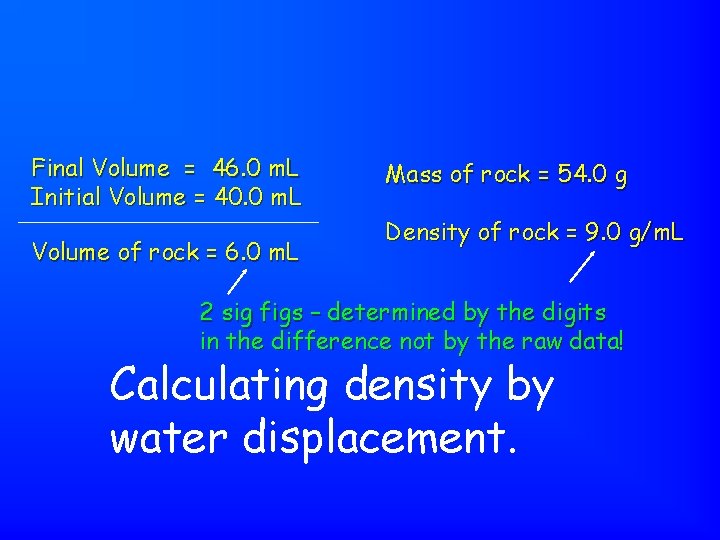
Final Volume = 46. 0 m. L Initial Volume = 40. 0 m. L Volume of rock = 6. 0 m. L Mass of rock = 54. 0 g Density of rock = 9. 0 g/m. L 2 sig figs – determined by the digits in the difference not by the raw data! Calculating density by water displacement.

58. 5 grams of Na. Cl = 1 mole. Calculated from P. T. 43. 875 g 1 mole 58. 5 grams = 0. 75000 moles/1. Liter = 0. 75 M 0. 8 M 1 sig fig 5 sig figs A solution contains 43. 875 grams of Na. Cl dissolved in 1. Liter of H 2 O. What is the molarity expressed to the correct sig figs?

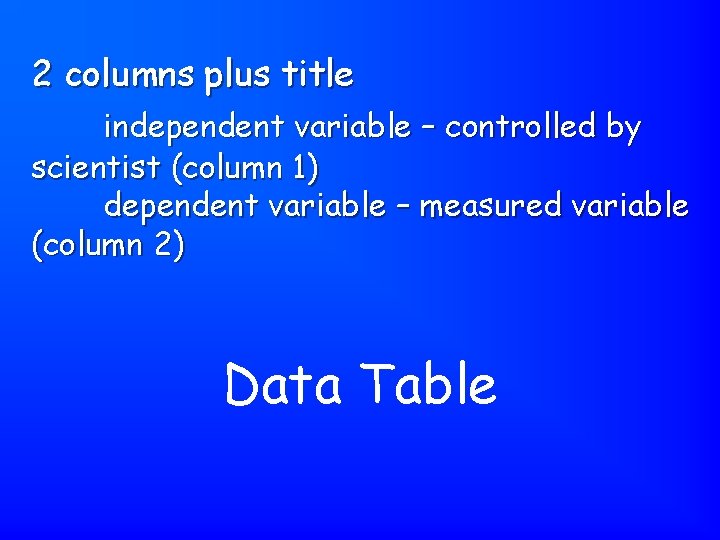
2 columns plus title independent variable – controlled by scientist (column 1) dependent variable – measured variable (column 2) Data Table

2 axes plus title independent variable – controlled by scientist (column 1) – GOES on X-AXIS dependent variable – measured variable (column 2) – GOES on Y-AXIS Graph

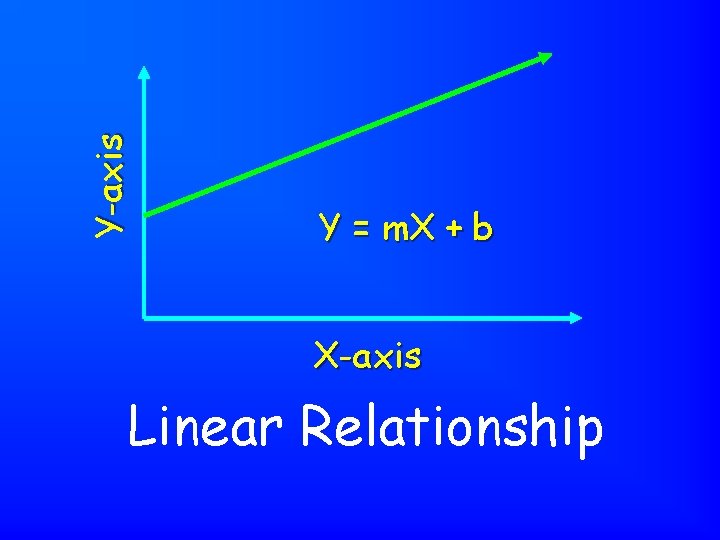
Y-axis Y = m. X + b X-axis Linear Relationship

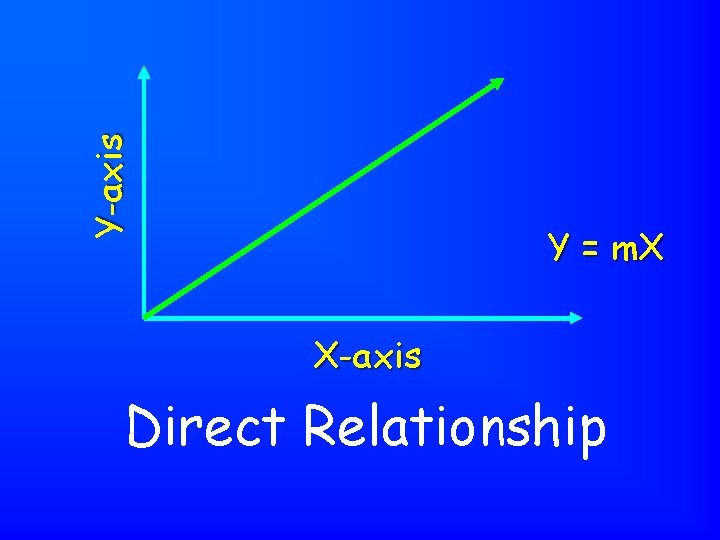
Y-axis Y = m. X X-axis Direct Relationship

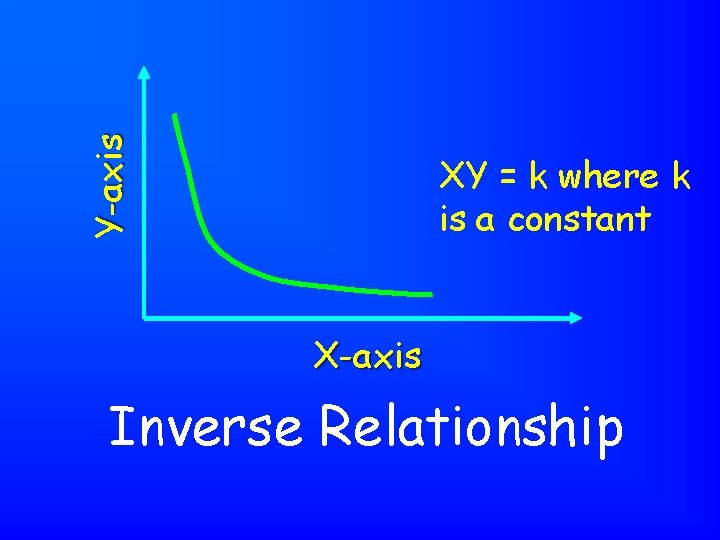
Y-axis XY = k where k is a constant X-axis Inverse Relationship

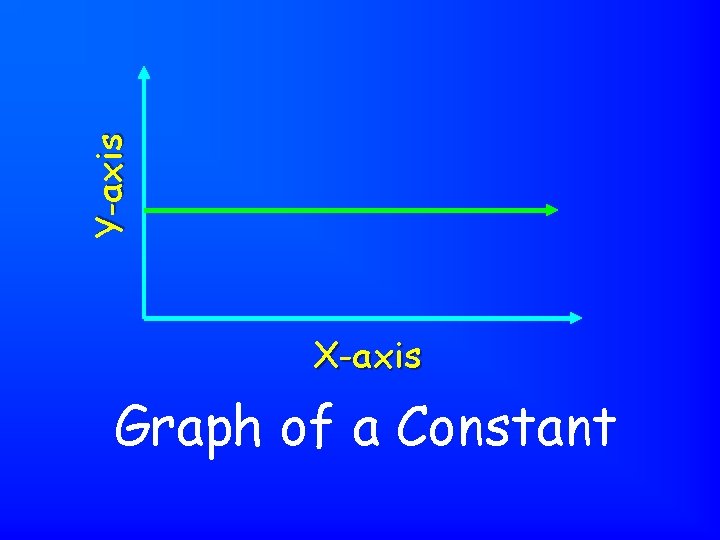
Y-axis X-axis Graph of a Constant