Matter matter anything that has mass and takes






- Slides: 20

Matter matter- anything that has mass and takes up space.

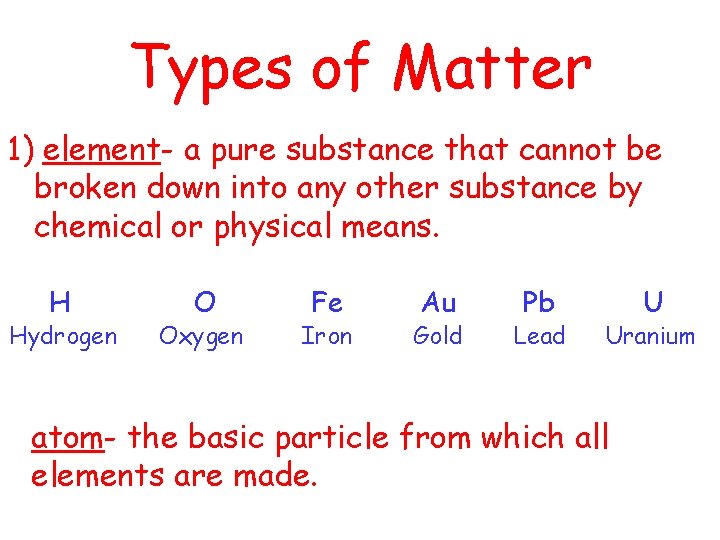
Types of Matter 1) element- a pure substance that cannot be broken down into any other substance by chemical or physical means. H Hydrogen O Oxygen Fe Iron Au Gold Pb Lead U Uranium atom- the basic particle from which all elements are made.

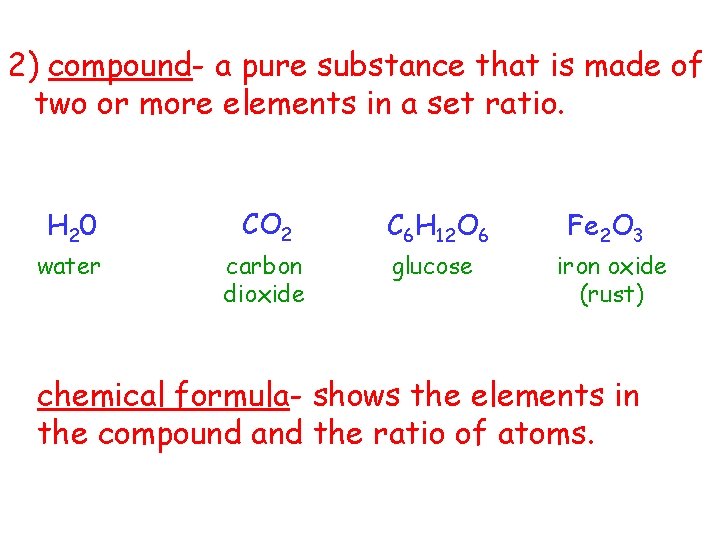
2) compound- a pure substance that is made of two or more elements in a set ratio. H 2 0 water CO 2 carbon dioxide C 6 H 12 O 6 glucose Fe 2 O 3 iron oxide (rust) chemical formula- shows the elements in the compound and the ratio of atoms.

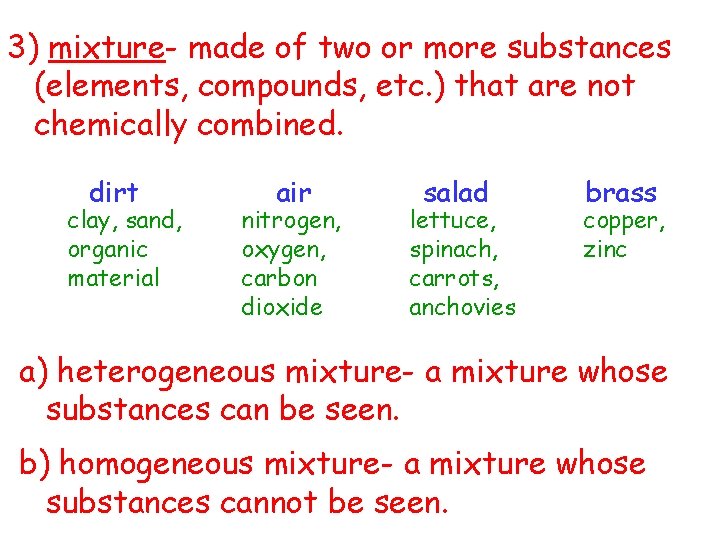
3) mixture- made of two or more substances (elements, compounds, etc. ) that are not chemically combined. dirt clay, sand, organic material air nitrogen, oxygen, carbon dioxide salad lettuce, spinach, carrots, anchovies brass copper, zinc a) heterogeneous mixture- a mixture whose substances can be seen. b) homogeneous mixture- a mixture whose substances cannot be seen.

Measuring Matter weight- the gravitational pull on an object. mass- the amount of matter in an object. weight can change with location, but mass remains the same. mass = 50 kg weight = 0 N weight = 490 N

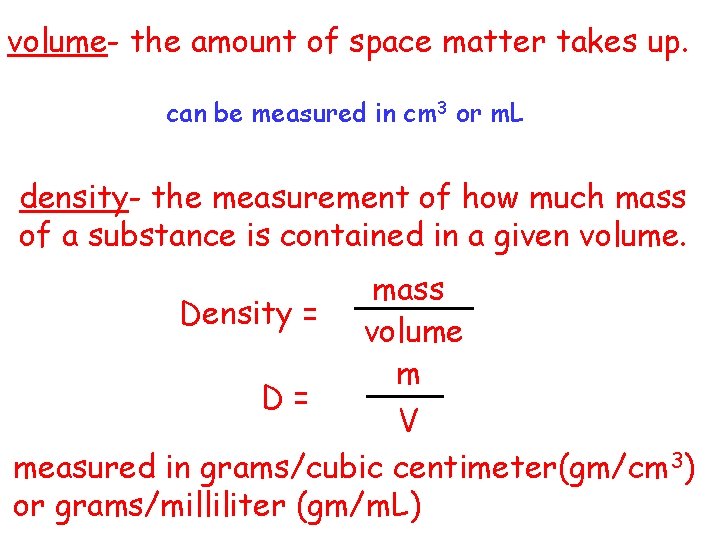
volume- the amount of space matter takes up. can be measured in cm 3 or m. L density- the measurement of how much mass of a substance is contained in a given volume. mass Density = volume m D= V measured in grams/cubic centimeter(gm/cm 3) or grams/milliliter (gm/m. L)

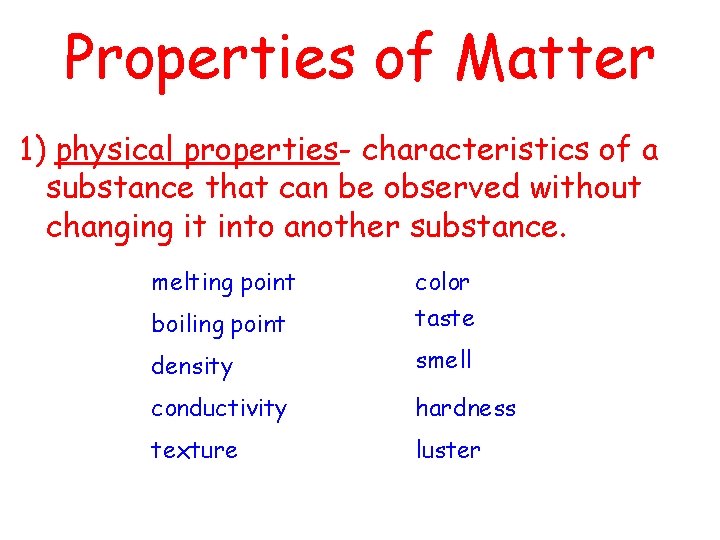
Properties of Matter 1) physical properties- characteristics of a substance that can be observed without changing it into another substance. melting point boiling point color taste density smell conductivity hardness texture luster

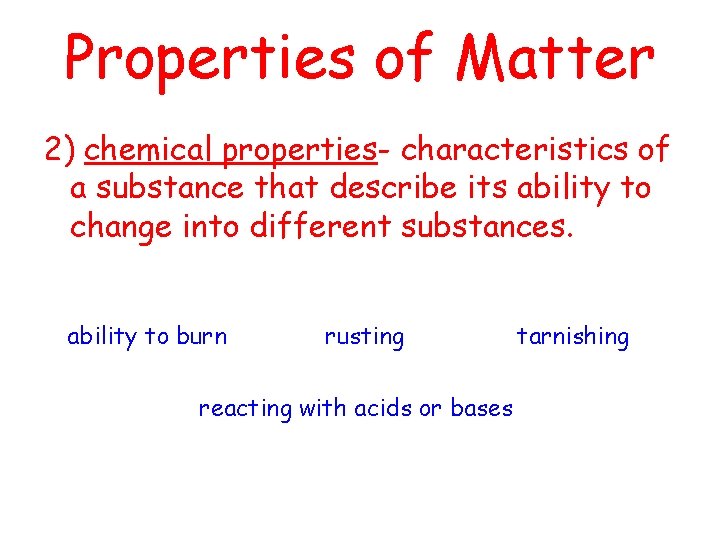
Properties of Matter 2) chemical properties- characteristics of a substance that describe its ability to change into different substances. ability to burn rusting reacting with acids or bases tarnishing

Changes in Matter 1) physical changes- a change that alters the form or appearance of a substance without changing it into another substance. melting boiling freezing evaporating

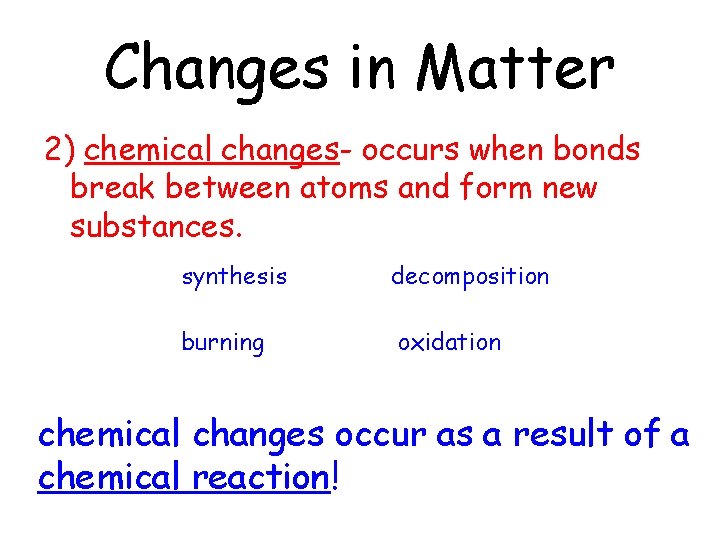
Changes in Matter 2) chemical changes- occurs when bonds break between atoms and form new substances. synthesis decomposition burning oxidation chemical changes occur as a result of a chemical reaction!

Law of Conservation of Mass states that: the total mass during a chemical or physical change remains constant or mass is neither created nor destroyed in a chemical or physical change.

Energy & Changes in Matter energy- the ability to do work or cause change. thermal energy- the total energy of all the particles in an object.

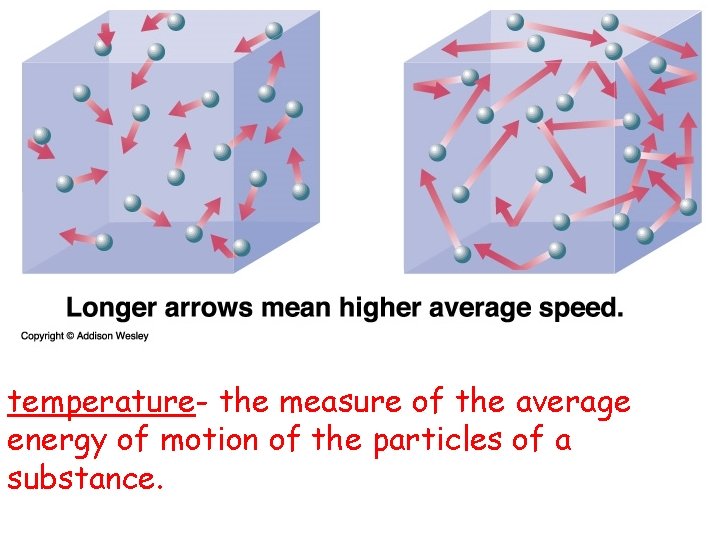
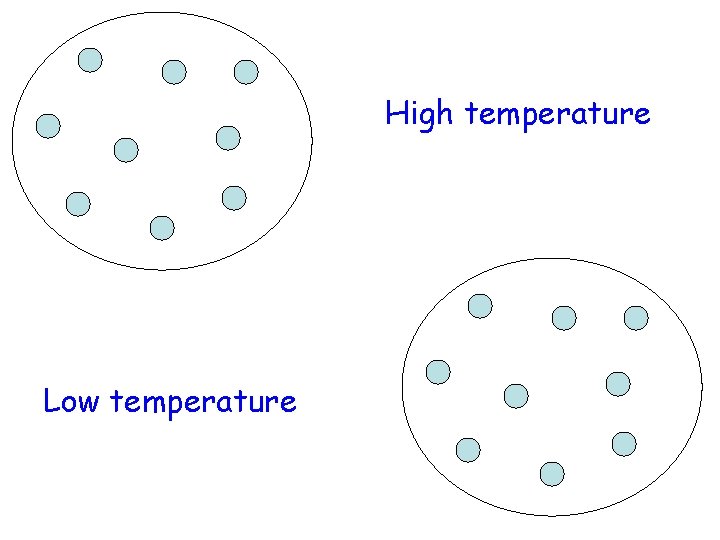
temperature- the measure of the average energy of motion of the particles of a substance.

High temperature Low temperature

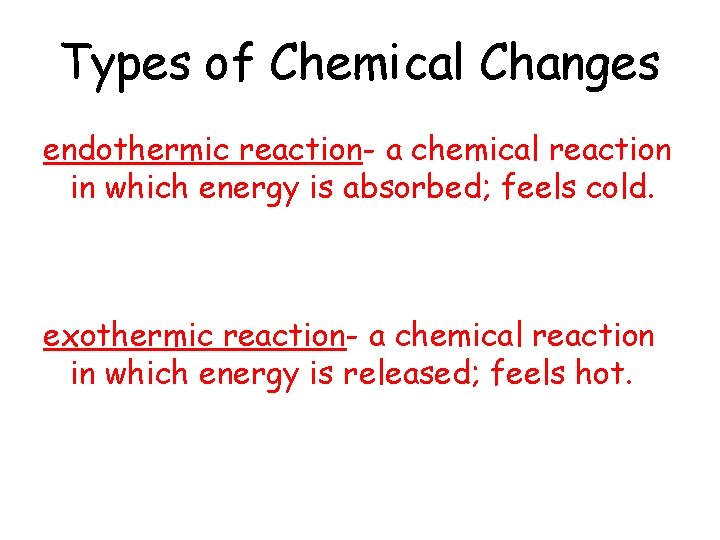
Types of Chemical Changes endothermic reaction- a chemical reaction in which energy is absorbed; feels cold. exothermic reaction- a chemical reaction in which energy is released; feels hot.

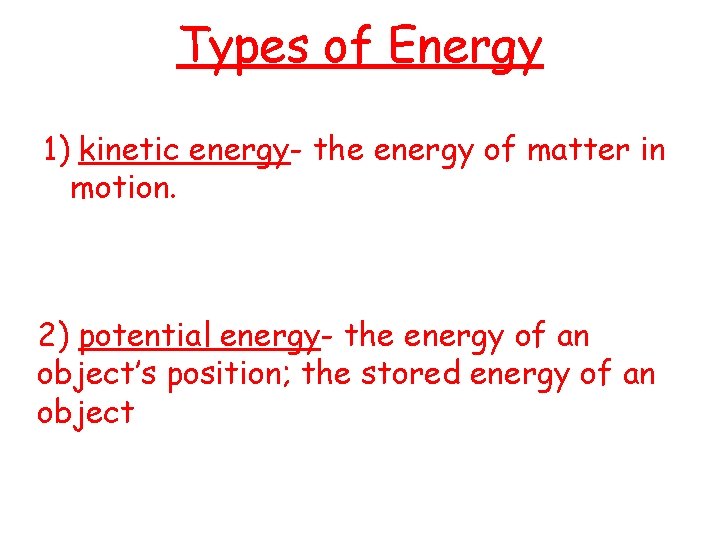
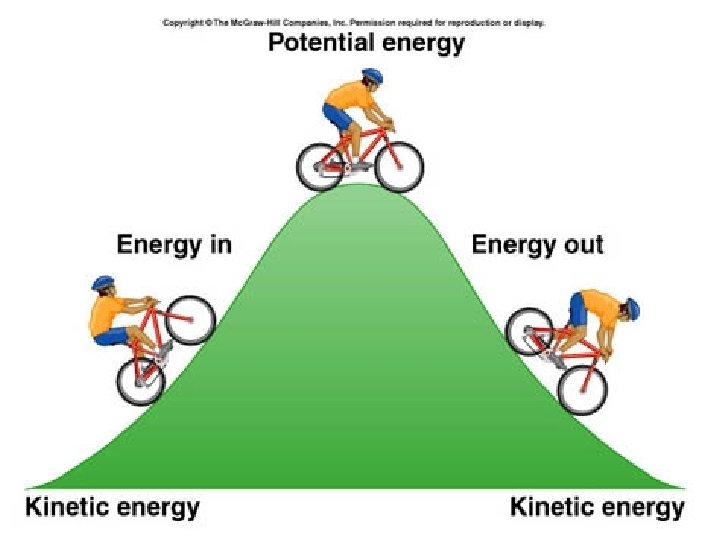
Types of Energy 1) kinetic energy- the energy of matter in motion. 2) potential energy- the energy of an object’s position; the stored energy of an object


3) Chemical energy- a form of potential energy that is stored in chemical bonds between atoms.

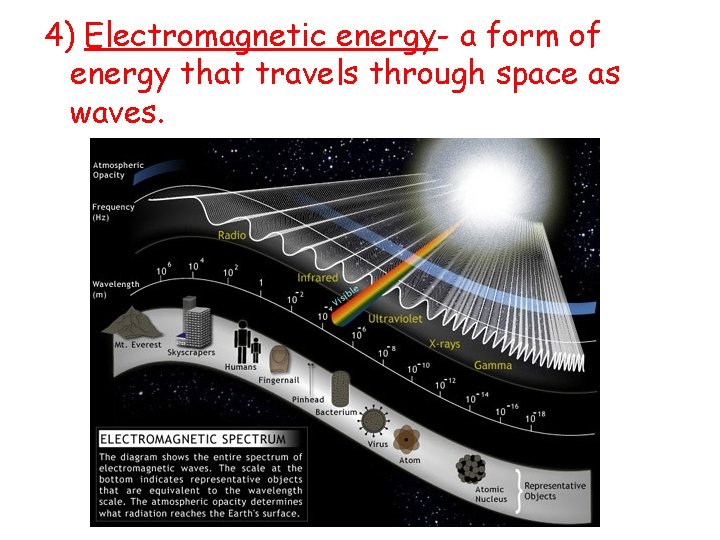
4) Electromagnetic energy- a form of energy that travels through space as waves.

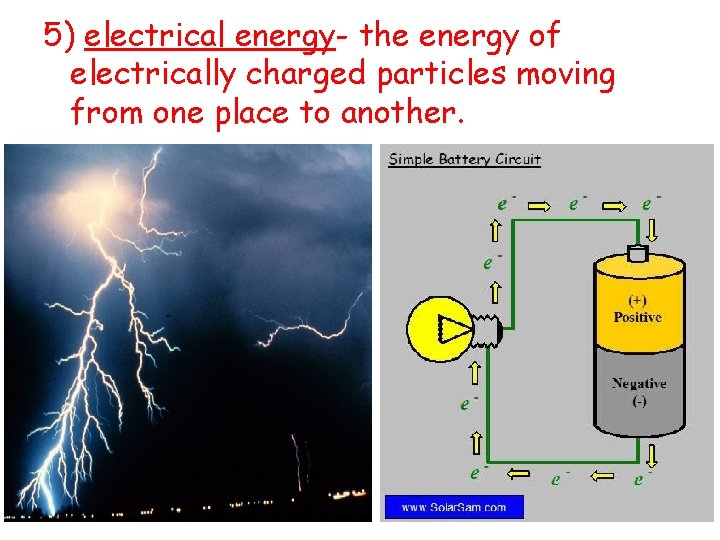
5) electrical energy- the energy of electrically charged particles moving from one place to another.
 Matter is anything that...
Matter is anything that... Matter is anything that has
Matter is anything that has Anything that has mass and take up space
Anything that has mass and take up space Anything that takes up space and has mass is
Anything that takes up space and has mass is Anything that takes up space and has mass is
Anything that takes up space and has mass is Defintion of matter
Defintion of matter Is anything that has mass and takes up space
Is anything that has mass and takes up space Anything that has mass or volume
Anything that has mass or volume Anthing that takes up space and has mass is called?
Anthing that takes up space and has mass is called? Matter defintion
Matter defintion Anything that takes up space
Anything that takes up space Compare and contrast template
Compare and contrast template What is anything that occupies space and has mass?
What is anything that occupies space and has mass? It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter has mass and occupies space
Matter has mass and occupies space Matter is anything that occupies space
Matter is anything that occupies space Phân độ lown
Phân độ lown Block xoang nhĩ độ 2
Block xoang nhĩ độ 2 Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật


































