Matter anything that has mass or takes up



























- Slides: 40


Matter § anything that has mass or takes up space

Properties and Changes in Matter All substances have characteristic properties used to distinguish between and separate them. -they may define an entire group (metals) -they may define a subgroup (sugars) -they can help to identify an unknown substance 3

Properties are either: 1. extensive- depend on the amount of matter present example: volume, 2. intensive- do not depend on amount of matter present example: boiling point IN: independent 12/6/2020 4

Properties of Matter § Two types of properties - chemical and physical § Which ones do you think are physical properties? Evolution of a gas : Chemical Cutting: Physical Color Change: Chemical

Physical Properties of Matter - a characteristic that can be observed or measured without changing the composition of the substance ex. viscosity, conductivity, malleability, melting point, boiling point, phase change

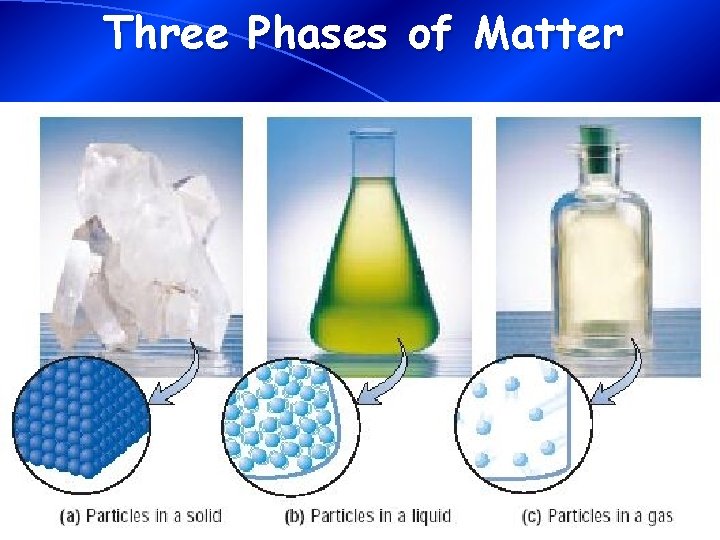
Phase Differences Solid – definite volume and shape; particles packed in fixed positions; particles are not free to move Liquid – definite volume but indefinite shape; particles close together but not in fixed positions; particles are free to move Gas – neither definite volume nor definite shape; particles are at great distances from one another; particles are free to move

Three Phases of Matter

Characteristics of Phase Changes § Phase Change - the reversible physical change that occurs when energy is either absorbed or released -absorbed: endothermic - released: exothermic § Common phase changes - freezing, melting, vaporization, condensation, sublimation, deposition

Melting - molecules are becoming less orderly - subatomic level: molecules gain energy and begin to vibrate ex. Ice (solid) Water (liquid)

Freezing - molecules are becoming more orderly - subatomic level: molecules lose energy and begin to slow down ex. water (liquid) ice (solid)

Vaporization -phase change in which a substance changes from a _____ into a _____. § § endothermic (absorbs energy) two processes boiling evaporation

Vaporization Cont. § Boiling - takes place throughout a liquid (boiling pt) - depends upon the atmospheric pressure - will differ for all substances ex. pot of water on the stove In Phoenix vs. Flagstaff § Evaporation - takes place at the surface of a liquid, occurs at temperatures below the boiling pt. ex. puddles after a rainy day within a few hours may disappear

Condensation - phase change in which a substance changes from a _______to a _______. - exothermic (gives off heat) ex. morning dew on grass water on mirror after a shower

Sublimation - phase change in which a substance changes from a solid to a gas/vapor without changing into a liquid first - endothermic (absorbs heat) ex. dry ice (solid carbon dioxide) vapors form clouds

Deposition - a gas/vapor changes directly into a solid without first changing to a liquid ex. dry ice: solid carbon dioxide water vapor ice when cold air hits window

Chemical Properties of Matter - any ability to produce a change in the composition of matter. - changing into a different substance § Flammability - material’s ability to burn in the presence of oxygen ex. newspaper, gasoline

Chemical Changes Cont. § How do you know if a chemical changed occurred? § Evidence of a chemical change - the evolution of a gas - the formation of a precipitate - the evolution or absorption of heat - emission of light - color change in the reaction system

Chemical vs. Physical Change § How do you know if it’s a physical or chemical change? - can be very tricky, they will both change some of the substances attributes - a chemical change IRREVERSIBLE CHANGE

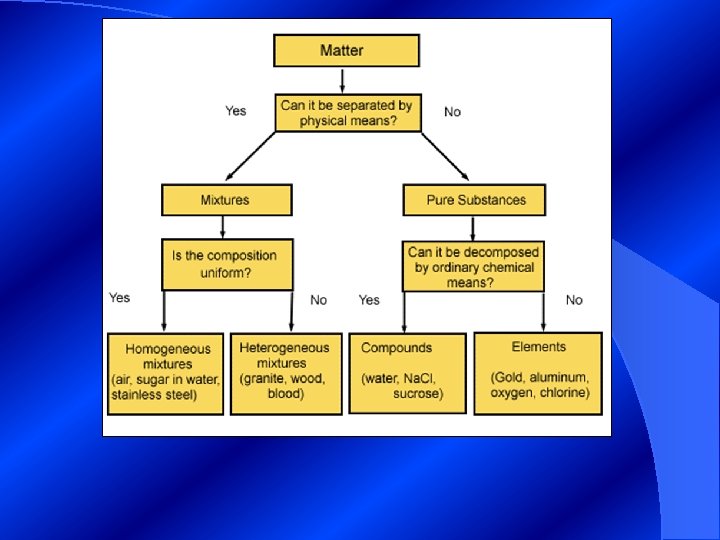
Classification of Matter § Matter exists as either a pure substance (element or compound) or a mixture. 12/6/2020 20

Pure Substances § the same composition throughout ex. table salt, sugar, sulfur, - every sample of a given substance has the same properties - 2 categories - elements ex. H, O, Si, C - compounds ex. Na. Cl, KBr

Elements § - a substance that can not be broken down into simpler substances - 119 elements - 88 are found naturally, about 90% - not equally common - others are made in laboratories

Elements Cont. - exception of hydrogen, and a few other trace elements are all remnants of stars that exploded long before our solar system came into existence - each element is represented by a symbol ex.

Elements Cont… - majority of the elements are not found in abundance - some are exceedingly rare - only a dozen or so make up everyday things - primarily: carbon, hydrogen, oxygen, nitrogen

Compounds - a substance made of atoms of more than one element bound together - unique and different from the elements it contains ex. Water: (H 2 O) liquid, clear, non toxic hydrogen - gas, colorless - non toxic - volatile & oxygen - gas, colorless - non toxic

Classifying Matter Cont. § Mixtures- blend of two or more kinds of pure substances, each of which retains its own identity and properties. § Parts are mixed physically and can usually be separated by physical means. § Two types of mixtures - heterogeneous - homogenous

Classifying Mixtures Cont. § Heterogeneous - not uniform in composition - you can see individual substances ex: Lucky Charms cereal § Suspension (Heterogeneous) - mixtures that separates into layers over time - suspended particles settle out of solution or are trapped by filter - larger particles can scatter light: will be cloudy ex. O. J. , sand/water, muddy water

Classifying Matter Cont. § Homogenous - substances are so evenly distributed that it is difficult to distinguish one substance from another -appears to contain only one substance ex. stainless steel: iron, nickel, chromium - 2 categories - solutions and colloids - based upon the size of the particles

Classifying Matter Cont. § Solutions (Homogenous) - mixtures that forms when substances dissolve - particles are too small to settle, scatter light, ex. salt water, windshield wiper fluid § Colloids (Homogeneous) - mixtures that contain some particles that are intermediate in size - do not separate into layers ex. homogenized milk vs. cow’s milk, fog

Suspensions and Colloids Suspensions: The particles are so large that they settle out of the solvent if not constantly stirred. Colloids: The particle is intermediate in size between those of a suspension and those of a solution.

Smog – A Suspension

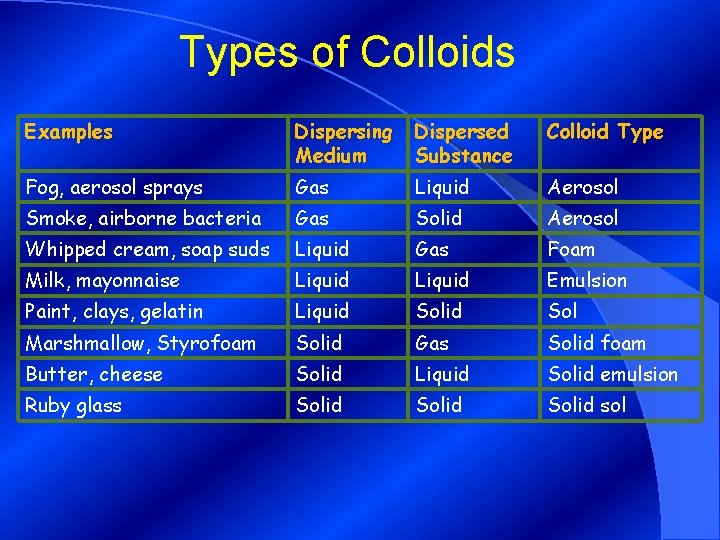
Types of Colloids Examples Dispersing Medium Dispersed Substance Colloid Type Fog, aerosol sprays Gas Liquid Aerosol Smoke, airborne bacteria Gas Solid Aerosol Whipped cream, soap suds Liquid Gas Foam Milk, mayonnaise Liquid Emulsion Paint, clays, gelatin Liquid Sol Marshmallow, Styrofoam Solid Gas Solid foam Butter, cheese Solid Liquid Solid emulsion Ruby glass Solid sol

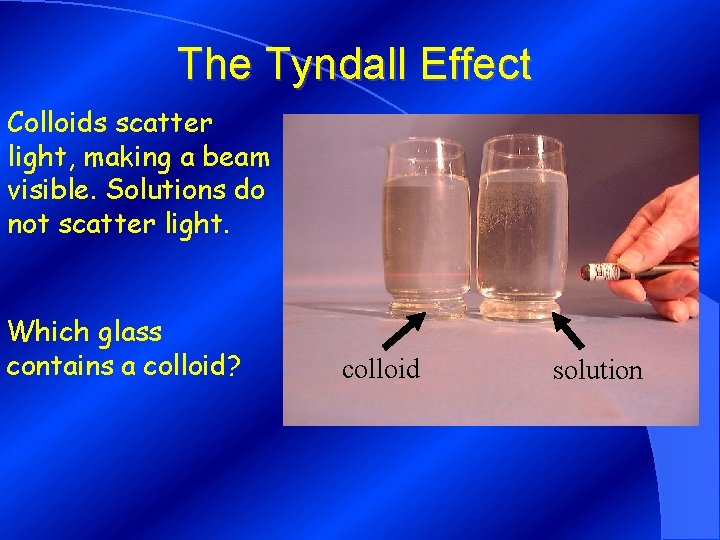
The Tyndall Effect Colloids scatter light, making a beam visible. Solutions do not scatter light. Which glass contains a colloid? colloid solution


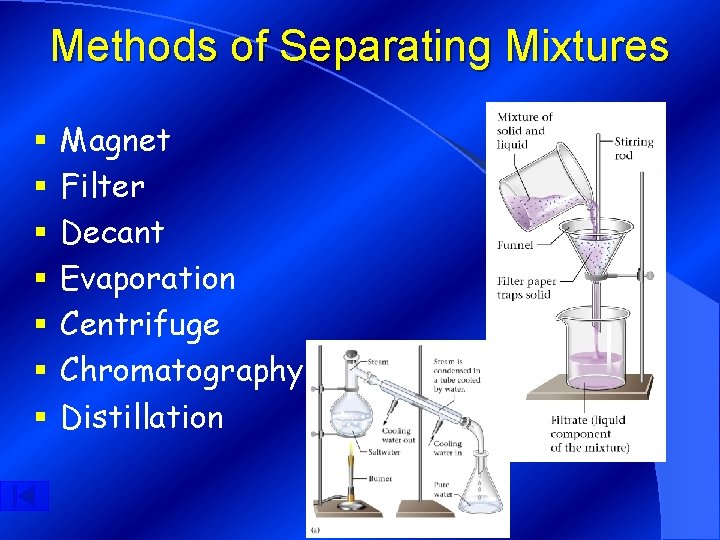
Methods of Separating Mixtures § § § § Magnet Filter Decant Evaporation Centrifuge Chromatography Distillation

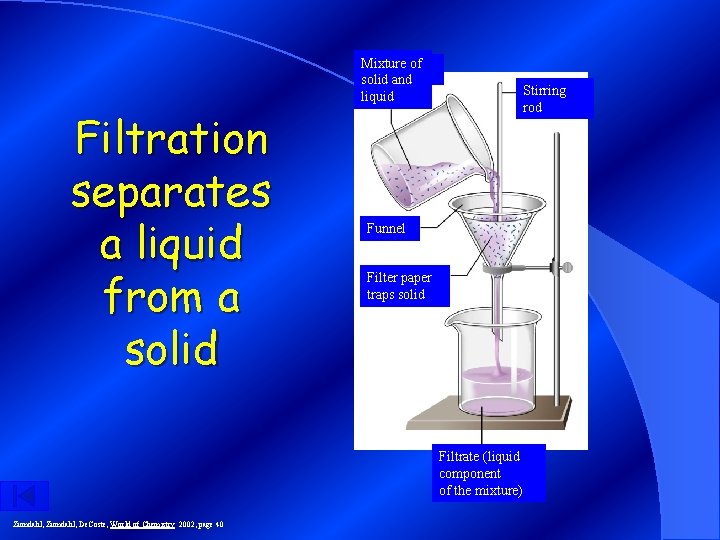
Filtration: Separates solid substances from liquids and solutions.

Mixture of solid and liquid Filtration separates a liquid from a solid Stirring rod Funnel Filter paper traps solid Filtrate (liquid component of the mixture) Zumdahl, De. Coste, World of Chemistry 2002, page 40

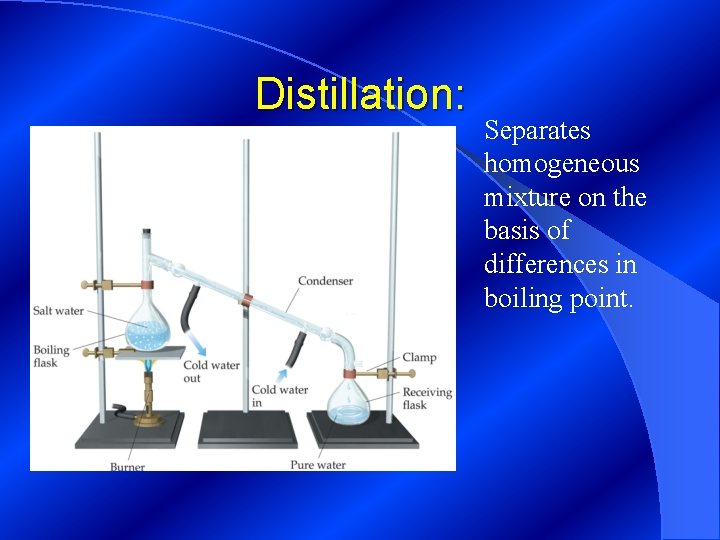
Distillation: Separates homogeneous mixture on the basis of differences in boiling point.

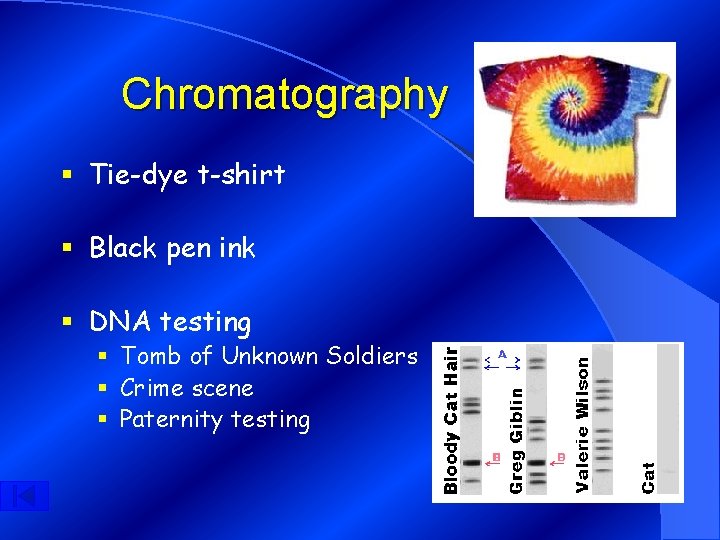
Chromatography § Tie-dye t-shirt § Black pen ink § DNA testing § Tomb of Unknown Soldiers § Crime scene § Paternity testing

Chromatography: Separates substances on the basis of differences in solubility in a solvent.