CHAPTER 3 MATTER Anything that occupies space and
















- Slides: 18

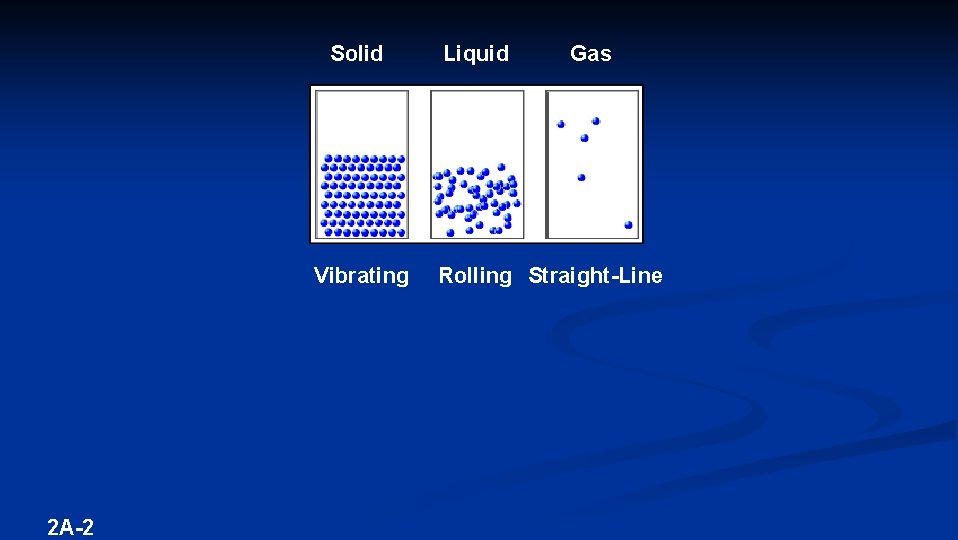
CHAPTER 3 – MATTER – Anything that occupies space and has mass Desk Rock Water Air You Matter is found in 3 phases or states: SOLID – definite shape and volume LIQUID – indefinite shape, definite volume GAS indefinite shape and volume – KINETIC MOLECULAR THEORY – Matter is composed of tiny particles that are in constant motion 2 A-1 (of 18) (3 – 5 -24 + 1 -4) (4 – 5 -16) (5 – 17 -18 + 1 -21)

Solid Vibrating 2 A-2 Liquid Gas Rolling Straight-Line

CHANGES IN MATTER PHYSICAL CHANGE – One that does not change the identity of the matter Tearing Crushing Freezing Melting Boiling CHEMICAL CHANGE – One that does change the identity of the matter, also called CHEMICAL REACTIONS Burning Rusting Tarnishing Spoiling Decomposing CHEMICAL ACTIVITY – The likelihood of matter to undergo chemical change ACTIVE - reacts vigorously INACTIVE - reacts slowly INERT - does not react 2 A-3

EVIDENCE FOR CHEMICAL CHANGE 1) Color change 2) A solid forms 3) Bubbles are formed 4) A flame is produces 5) Heat is absorbed or released 2 A-4

TESTS FOR GASES Hydrogen - makes a burning splint “pop” Oxygen - makes a glowing splint ignite Carbon dioxide - puts out a burning splint 2 A-5

TYPES OF MATTER SUBSTANCE – A homogeneous material consisting of one kind of matter Water Iron Gold Nitrogen Salt There are 2 types of substances ELEMENT – A substance that cannot be broken down into simpler substances by chemical means Iron Gold Nitrogen (Found on the Periodic Table) COMPOUND – A substance that can be broken down into simpler substances by chemical means Water Salt 2 A-6

Water Compound ↓electricity Hydrogen and Oxygen 2 A-7 Elements Compound Elements Salt ↓electricity Sodium and Chlorine

MIXTURE – A physical combination of substances with a variable composition Granite Air Seawater Wine Spanish Omelet There are 2 types of mixtures HETEROGENEOUS – A mixture that has parts with visibly different properties Granite Spanish Omelet HOMOGENEOUS – A mixture that has the same properties throughout Air Seawater SOLUTION – A homogeneous mixture 2 A-8 Wine

PROPERTIES OF COMPOUNDS 1) Components (elements) combine chemically 2) Components (elements) are in a definite proportion 3) Components lose their characteristic properties 4) Components can only be separated by chemical means + 2 g Sodium 4 g Sodium 2 A-9 3 g Chlorine 6 g Chlorine 5 g Sodium chloride 10 g Sodium chloride

PROPERTIES OF MIXTURES 1) Components (elements or compounds) combine physically 2) Components (elements or compounds) can be in any proportions 3) Components retain their characteristic properties 4) Components can be separated by physical means + 2 g Tin 2 A-10 3 g Copper 6 g Copper Bronze Still Bronze

SEPARATION OF MIXTURES DISTILLATION – Separating the substances in a mixture by vaporizing (boiling) one of them FILTRATION – Separating the substances in a mixture if one is a solid and one is a liquid 2 A-11

Matter Mixture Heterogeneous 2 A-12 Homogeneous Substance Compound Element

CHAPTER 10 – THE CONCEPT OF ENERGY – The capacity to do work Units of energy: CALORIE (cal) – The energy needed to raise the temperature of 1 gram of water 1 Cº JOULE (J) – The energy needed to accelerate a 1 kg object 1 m/s 2 over a 1 m distance 1 cal = 4. 184 J 2 A-13

Convert 25. 0 calories to joules 25. 0 cal x 4. 184 J _____ 1 cal 2 A-14 = 105 J

SPECIFIC HEAT CAPACITY (s) – The energy needed to raise the temperature of 1 gram of a substance 1 Cº Substance Water Sand Iron 2 A-15 Specific Heat Capacity (s) 4. 184 J/g. Cº 0. 45 J/g. Cº

To calculate the energy needed to raise the temperature of a substance: Q = smΔT Q s m ΔT = = 2 A-16 energy (J) specific heat capacity (J/g. Cº) mass of the substance (g) temperature change (Cº)

Calculate the energy needed to raise the temperature of 50. 0 g of water from 22ºC to 37ºC. Q = smΔT = (4. 184 J/g. Cº)(50. 0 g)(15 Cº) = 3, 138 J = 3, 100 J 2 A-17

A 2. 3 g sample of gold requires 6. 0 J of energy to raise its temperature from 25ºC to 45ºC. Find gold’s specific heat capacity. Q = smΔT Q ______ mΔT = s 6. 0 J = _________ (2. 3 g)(20. Cº) 2 A-18 = 0. 13 J/g. Cº