Matter What is it Matter Anything that occupies




![Consider this……. . If an atom is a marker [if atom=marker] Then an element Consider this……. . If an atom is a marker [if atom=marker] Then an element](https://slidetodoc.com/presentation_image_h2/46cbb1cf654e2d7066b6448a492cc5d4/image-8.jpg)







![If I had a block of pure gold (woohoo!) [an element] OR a spoonful If I had a block of pure gold (woohoo!) [an element] OR a spoonful](https://slidetodoc.com/presentation_image_h2/46cbb1cf654e2d7066b6448a492cc5d4/image-20.jpg)



























- Slides: 56

Matter…. . What is it? Matter: Anything that occupies space and has mass

What is Matter? • It is: – Anything you can hold or touch – It has mass – It has volume – Us! – Our Earth • It is not: – Light – Sound – Electricity

Why does matter MATTER? Because we’re going to learn about chemistry…. And Chemistry = The study of matter and changes in matter This could be you!!

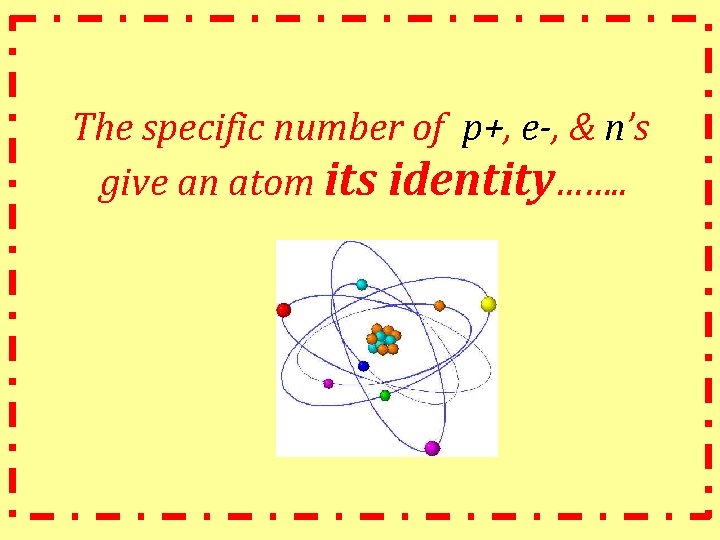
So what is matter made up of…. . ? At it’s most basic level……… Atoms! Atom: smallest unit of matter -made up of protons, electrons, neutrons -everything (classified as matter) is made up of atoms!

The specific number of p+, e-, & n’s give an atom its identity……. .

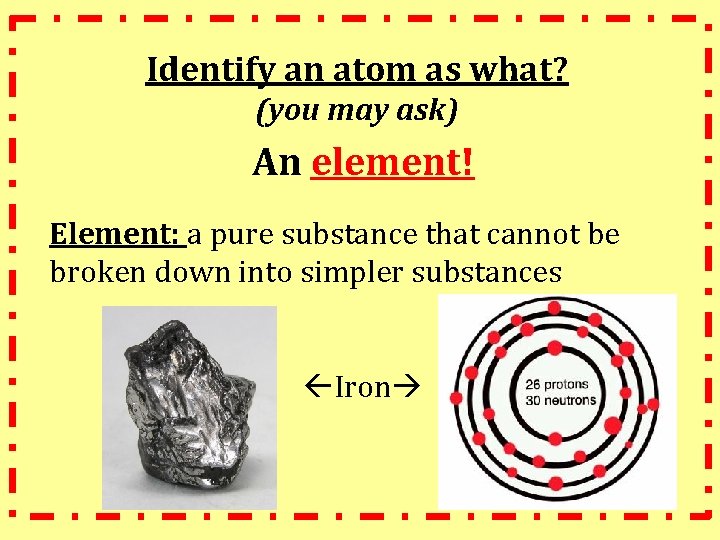
Identify an atom as what? (you may ask) An element! Element: a pure substance that cannot be broken down into simpler substances Iron

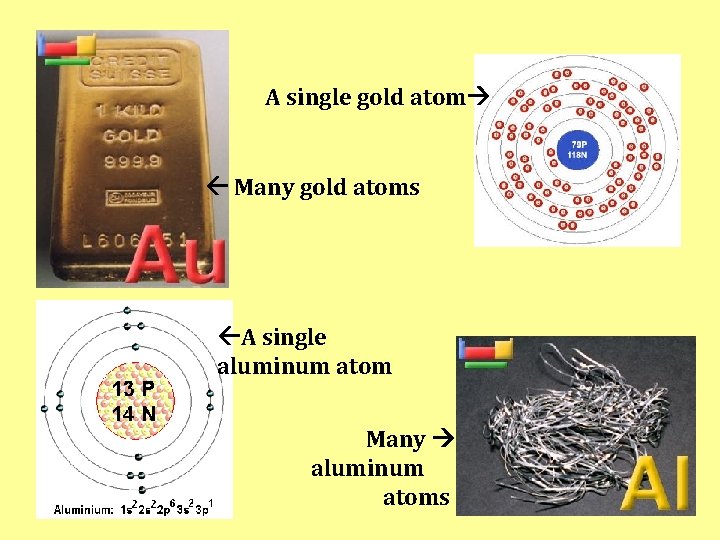
A single gold atom Many gold atoms A single aluminum atom Many aluminum atoms
![Consider this If an atom is a marker if atommarker Then an element Consider this……. . If an atom is a marker [if atom=marker] Then an element](https://slidetodoc.com/presentation_image_h2/46cbb1cf654e2d7066b6448a492cc5d4/image-8.jpg)
Consider this……. . If an atom is a marker [if atom=marker] Then an element could be a red marker [then element=red marker]

Now you try…… If an atom is a vegetable? Then an element could be a _______ If an atom is a pie? Then an element could be a _______

Science Theater!!!! What if I put a whole bunch of atoms together? . . If they’re the same, they’re an ELEMENT If they’re different, they’re a……………

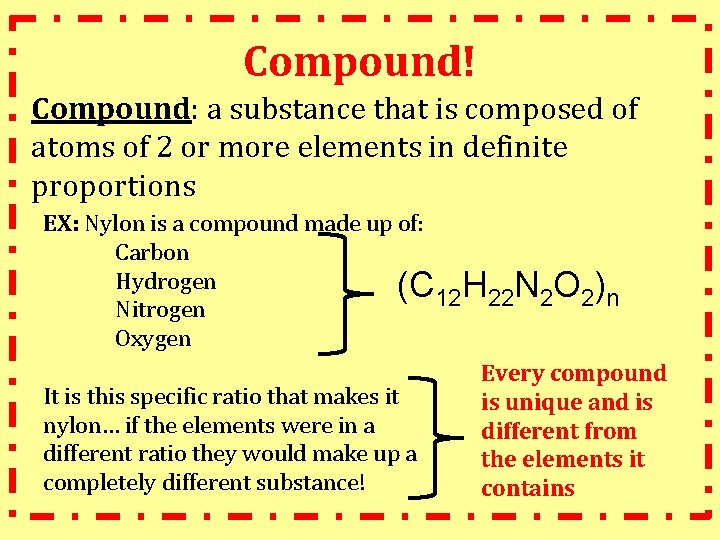
Compound! Compound: a substance that is composed of atoms of 2 or more elements in definite proportions EX: Nylon is a compound made up of: Carbon Hydrogen (C 12 H 22 N 2 O 2)n Nitrogen Oxygen Every compound It is this specific ratio that makes it is unique and is nylon… if the elements were in a different from different ratio they would make up a the elements it completely different substance! contains

I showed Nylon as: (C 12 H 22 N 2 O 2) This is a representation/symbolic form of a compound…. Any idea what we call it?

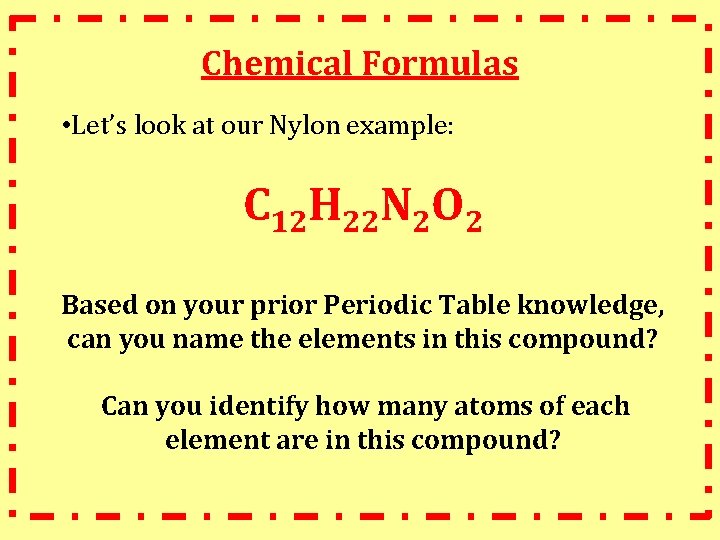
Chemical Formula! Chemical Formula: symbolic representation of the components of a compound • made up of element symbols and numbers (subscripts) that display the ratio of atoms • Let’s look at our Nylon example:

INTERMISSION Take a lap around the room

Chemical Formulas • Let’s look at our Nylon example: C 12 H 22 N 2 O 2 Based on your prior Periodic Table knowledge, can you name the elements in this compound? Can you identify how many atoms of each element are in this compound?

C 12 H 22 N 2 O 2 Carbon : 12 Hydrogen : 22 Nitrogen : 2 Oxygen : 2

Element Symbols C 12 H 22 N 2 O 2 # of atoms in compound (of that element) Notice how the numbers come after the letter they’re describing

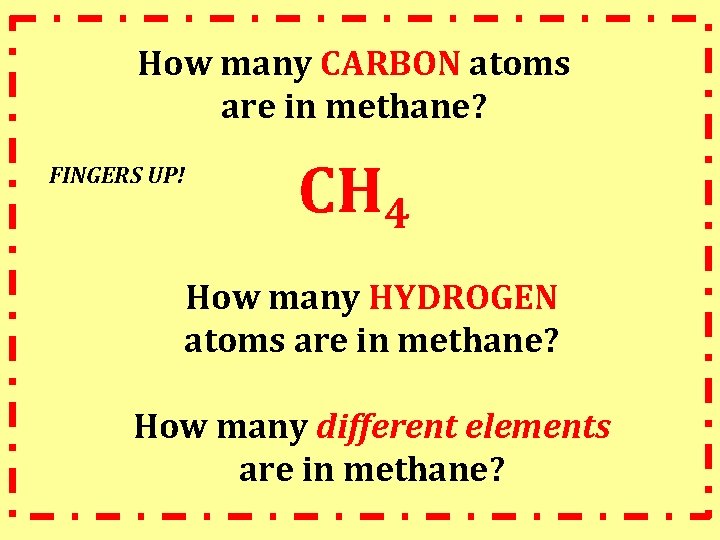
How many CARBON atoms are in methane? FINGERS UP! CH 4 How many HYDROGEN atoms are in methane? How many different elements are in methane?

How many different elements are in sodium bromide? FINGERS UP! Na. Br Sodium Bromine How many SODIUM (Na) atoms are in sodium bromide? How many BROMINE (Br) atoms are in sodium bromide?
![If I had a block of pure gold woohoo an element OR a spoonful If I had a block of pure gold (woohoo!) [an element] OR a spoonful](https://slidetodoc.com/presentation_image_h2/46cbb1cf654e2d7066b6448a492cc5d4/image-20.jpg)
If I had a block of pure gold (woohoo!) [an element] OR a spoonful of sugar [a compound] I would call these Pure Substances

Whereas, if I were in the ocean (i. e. salt water) [2 different compounds, specifically 2 different pure substances] I would call the ocean a…. MIXTURE

Pure substance: Any matter that has a FIXED composition and definite properties -Pure elements or compounds -EX. Gold, iron, salt, sugar Mixture: Combination of 2 or more substances in which the substances retain their distinct identities -EX. Salt water, balsamic and oil dressing

There are various types of mixtures, specifically 2 important ones…. Any ideas what they are? Homogeneous & Heterogeneous What’s the difference?

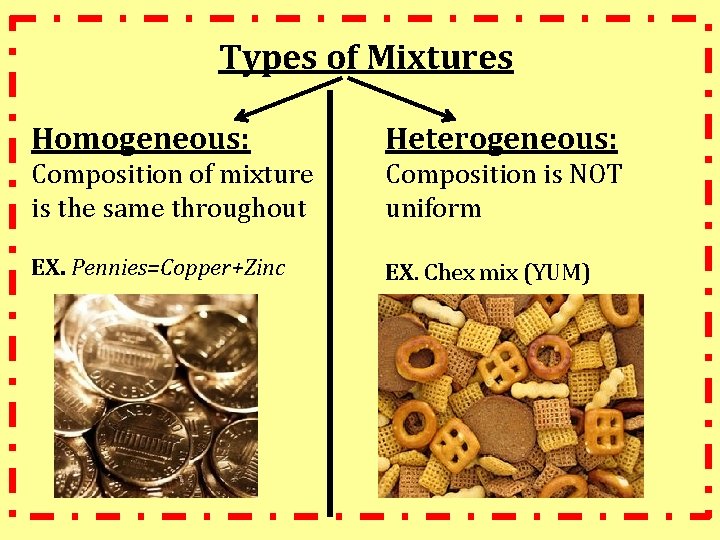
Types of Mixtures Homogeneous: Heterogeneous: Composition of mixture is the same throughout Composition is NOT uniform EX. Pennies=Copper+Zinc EX. Chex mix (YUM)

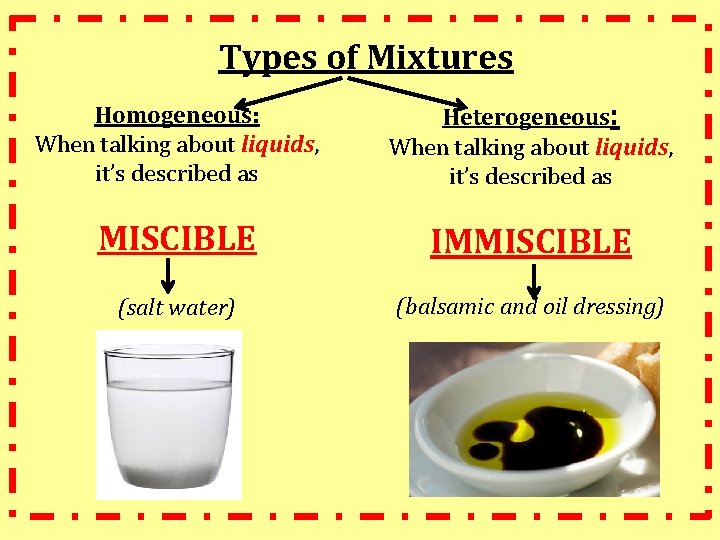
Types of Mixtures Homogeneous: When talking about liquids, it’s described as Heterogeneous: When talking about liquids, it’s described as MISCIBLE IMMISCIBLE (salt water) (balsamic and oil dressing)

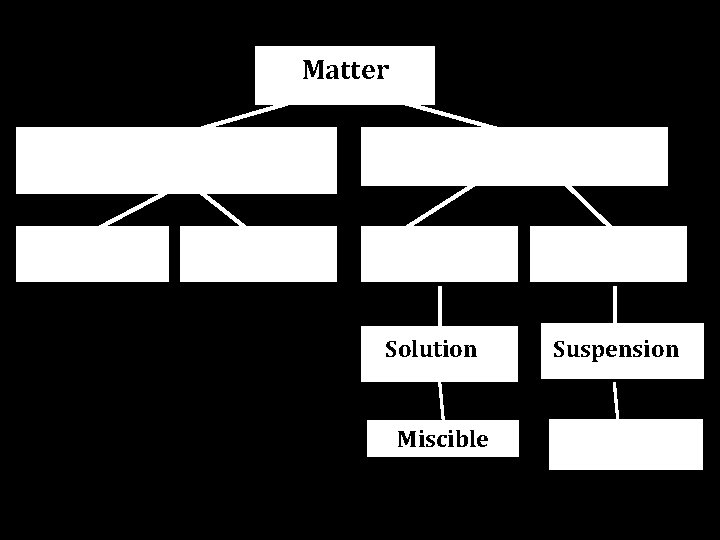
Matter Solution Miscible Suspension

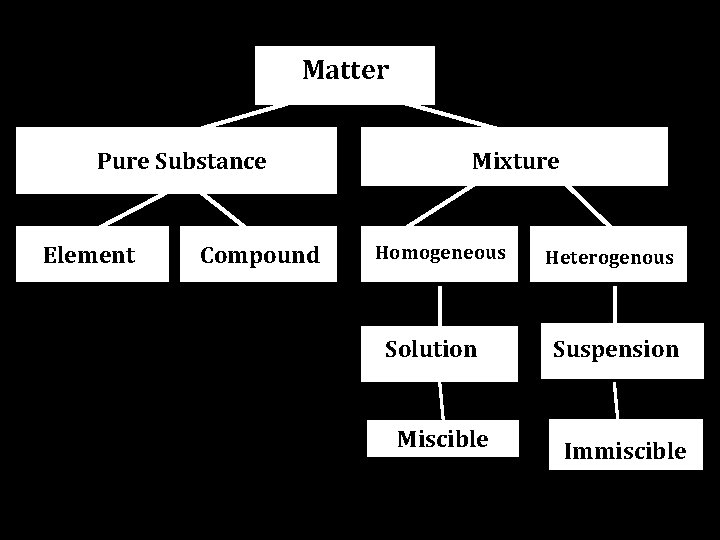
Matter Pure Substance Element Compound Mixture Homogeneous Solution Miscible Heterogenous Suspension Immiscible

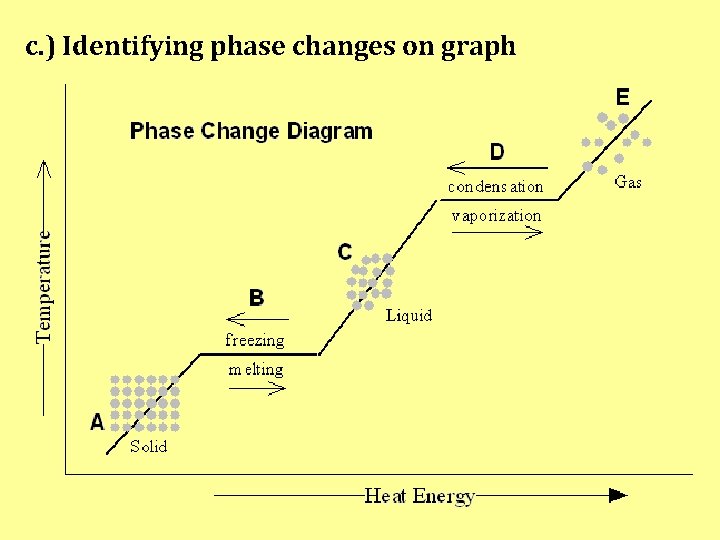
c. ) Identifying phase changes on graph

Over the course of the unit the words “Physical”, “Chemical” and “Properties” have come up a BUNCH! Let’s find out what they mean Take out your Notes

2 main types of PROPERTIES: a. ) Physical Properties: characteristics that can be observed or measured without changing the composition of the substance ex. ) texture, color, boiling pt, density Ex. ) Dirt=dark brown, grainy….

2 main types of PROPERTIES: b. ) Chemical Properties: characteristics that are only measurable and observable during a reaction; changing this type of property would change the identity of the substance ex. ) reactivity, p. H Sodium+water----->

**Physical and Chemical properties are IMPORTANT because they can help us identify different substances based on their properties** The more properties we can identify for a substance, the better we know the nature of that substance. These properties can then help us model the substance and thus understand how this substance will behave under various conditions.

For example, we know (based on many, many experiments) that water - has a boiling point of 100°C - has density of 1 g/m. L - is not flammable If I tested Mystery Liquid A & it…. . -boiled at 100°C -had a density of 1 g/m. L -did not light on fire when a match was What could you INFER based on these OBSERVATIONS?

If I tested Mystery Liquid A & it…. . -boiled at 100°C -had a density of 1 g/m. L -did not light on fire when a match was Then…. You could INFER that Mystery Liquid A is WATER based on these OBSERVATIONS

On the other hand, If I tested Mystery Liquid A & it…. . -boiled at 180°C -had a density of 0. 25 g/m. L -did not light on fire when a match was Could you INFER that Mystery Liquid A is water based on these OBSERVATIONS? NO (we’d have to compare our data to other known information and try to figure out what liquid it is)

2 main types of CHANGES: Physical Change: a change that does not alter the chemical composition of a substance [Δ that ≠ change chem. comp. of subst. ] ex. ) cutting paper, ice melting, dissolving sugar in water

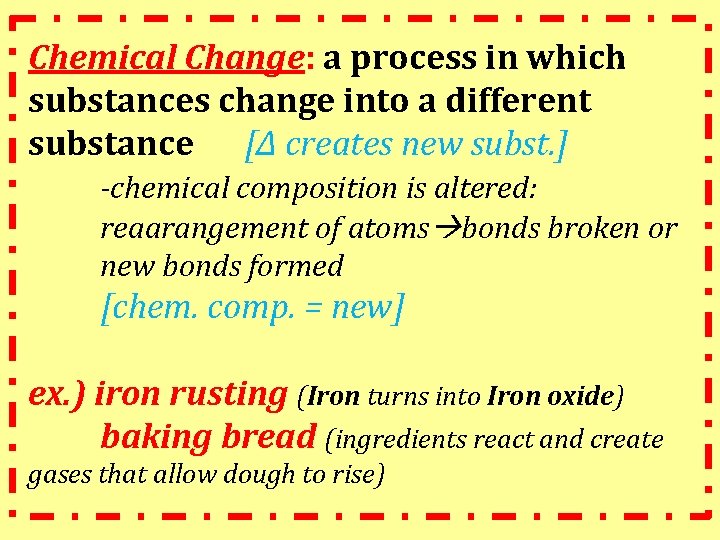
Chemical Change: a process in which substances change into a different substance [Δ creates new subst. ] -chemical composition is altered: reaarangement of atoms bonds broken or new bonds formed [chem. comp. = new] ex. ) iron rusting (Iron turns into Iron oxide) baking bread (ingredients react and create gases that allow dough to rise)

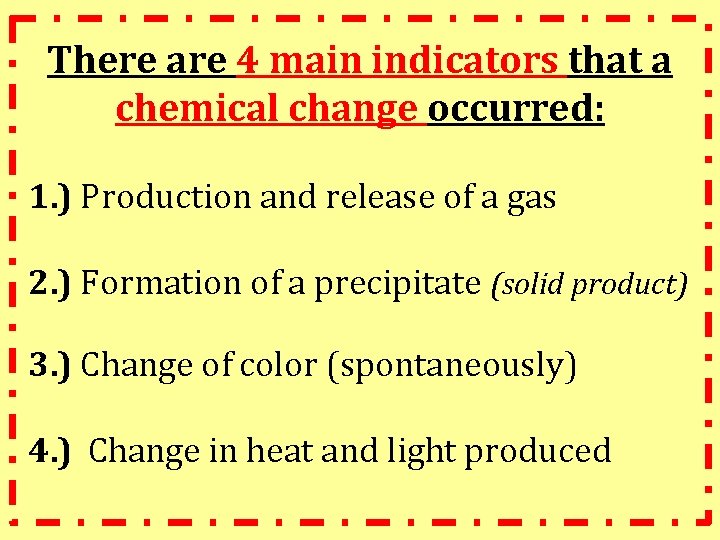
There are 4 main indicators that a chemical change occurred: 1. ) Production and release of a gas 2. ) Formation of a precipitate (solid product) 3. ) Change of color (spontaneously) 4. ) Change in heat and light produced

Color 1. ) Physical Property 2. ) Chemical Property

Odor 1. ) Physical Property 2. ) Chemical Property

Reactivity 1. ) Physical Property 2. ) Chemical Property

Boiling Point 1. ) Physical Property 2. ) Chemical Property

Density 1. ) Physical Property 2. ) Chemical Property

Now let’s try it with physical and chemical CHANGES (∆’s)

Boiling of Water 1. ) Physical Change (∆) 2. ) Chemical Change (∆)

Tearing of Clothes 1. ) Physical ∆ 2. ) Chemical ∆

Rusting of a nail 1. ) Physical ∆ 2. ) Chemical ∆

Melting of Ice Cream 1. ) Physical ∆ 2. ) Chemical ∆

Metabolizing Food 1. ) Physical ∆ 2. ) Chemical ∆

Dissolving Kool-Aid in H 2 O 1. ) Physical ∆ 2. ) Chemical ∆

Wood burning in a fireplace 1. ) Physical ∆ 2. ) Chemical ∆

MOLECULES For the sake of our class, we’ll use compounds and molecules somewhat interchangeably but they, ultimately, are NOT THE SAME Write this down: Molecule ≠ Compound

MOLECULES Molecule: a group of atoms held together by chemical bonds Ex. ) CH 3 OH methanol C 6 H 12 O 6 glucose C 2 H 5 OH ethanol

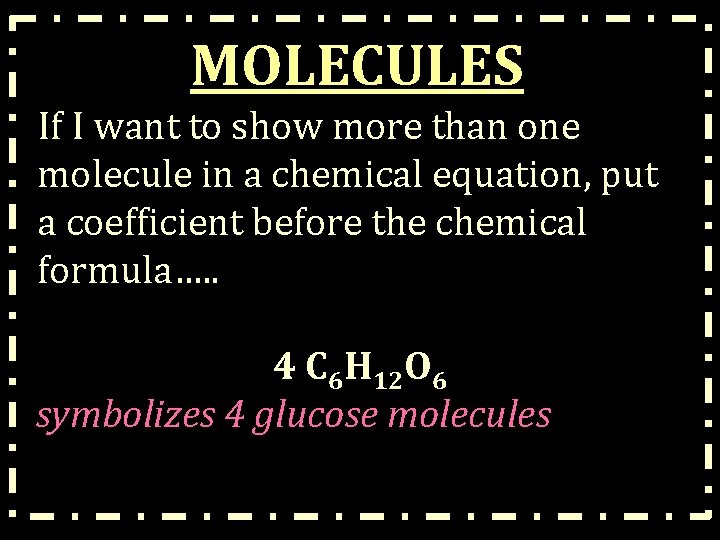
MOLECULES If I want to show more than one molecule in a chemical equation, put a coefficient before the chemical formula…. . 4 C 6 H 12 O 6 symbolizes 4 glucose molecules

How many Carbon atoms are in 1 molecule of glucose? 4 C 6 H 12 O 6 How many Carbon atoms are in 4 molecules of glucose?

How many atoms are in 1 molecule of glucose? 4 C 6 H 12 O 6 How many atoms are in 4 molecules of glucose?
 Has mass and occupies space
Has mass and occupies space Matter is anything that has mass and occupies space
Matter is anything that has mass and occupies space Matter is anything that occupies
Matter is anything that occupies It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter has mass and occupies space
Matter has mass and occupies space Block nhĩ thất độ 3
Block nhĩ thất độ 3 Tìm vết của đường thẳng
Tìm vết của đường thẳng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Tôn thất thuyết là ai
Tôn thất thuyết là ai Phân độ lown
Phân độ lown Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Matter is anything that has
Matter is anything that has Matter is defined as anything that
Matter is defined as anything that Matter anything that
Matter anything that Matter is anything that
Matter is anything that Matter is anything that?
Matter is anything that? Mass vs weight
Mass vs weight Matter is anything that:
Matter is anything that: Matter is anything that has mass and volume.
Matter is anything that has mass and volume. Benjamin cummings
Benjamin cummings Matter anything that
Matter anything that Anything that has mass
Anything that has mass What is matter
What is matter Matter vs mass
Matter vs mass All matter has and takes up
All matter has and takes up Matter anything that
Matter anything that Is oil more dense than water
Is oil more dense than water Is anything that has mass and takes up space.
Is anything that has mass and takes up space. No matter anything
No matter anything Malleability defintion
Malleability defintion Matter is anything that has
Matter is anything that has Matter anything that
Matter anything that K=pv
K=pv Trophic
Trophic What is k in boyle's law
What is k in boyle's law A gas occupies 7.84 cm3
A gas occupies 7.84 cm3 Within the meninges cerebrospinal fluid occupies the
Within the meninges cerebrospinal fluid occupies the A sample of neon gas occupies a volume of 677 ml at 134 kpa
A sample of neon gas occupies a volume of 677 ml at 134 kpa A gas occupies 473 cm3 at 36°c. find its volume at 94°c
A gas occupies 473 cm3 at 36°c. find its volume at 94°c Characteristics of gases
Characteristics of gases Divine right theory government
Divine right theory government Boyle's gas law formula
Boyle's gas law formula Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Rhinencephalon
Rhinencephalon Section 1 composition of matter
Section 1 composition of matter Brain falx
Brain falx Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Grey matter nervous system
Grey matter nervous system Composition of matter section 1
Composition of matter section 1 Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter What is hippolyta’s reaction to the play?
What is hippolyta’s reaction to the play? Abundant compound
Abundant compound