Matter Matter Anything that has mass and occupies









- Slides: 15

Matter

Matter Anything that has mass and occupies space – Mass measured in grams or kilograms – Space/volume measured in liters or cm 3

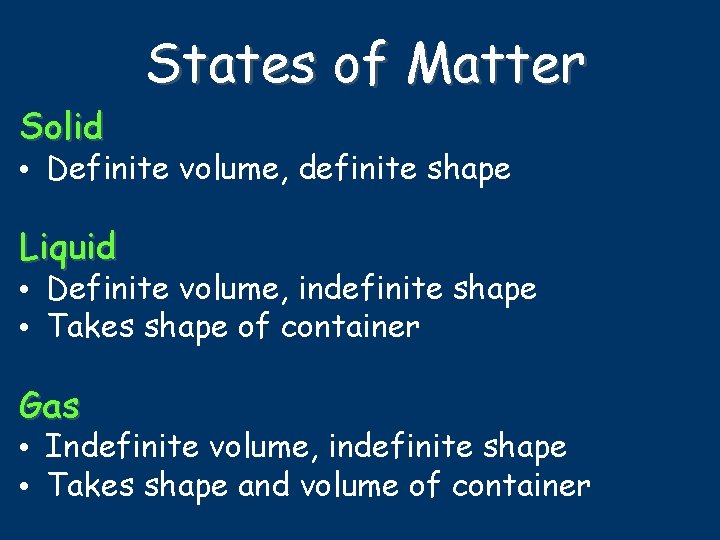
Solid States of Matter • Definite volume, definite shape Liquid • Definite volume, indefinite shape • Takes shape of container Gas • Indefinite volume, indefinite shape • Takes shape and volume of container

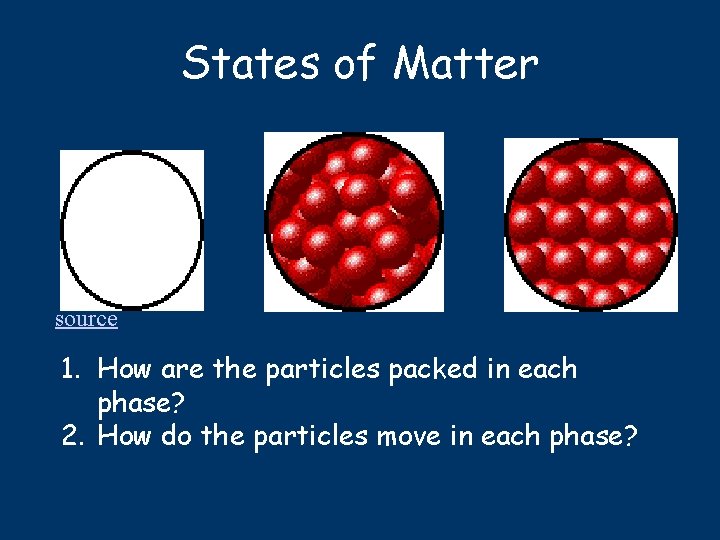
States of Matter source 1. How are the particles packed in each phase? 2. How do the particles move in each phase?

Physical Properties Describe appearance: Words: - color - texture - luster - odor - state: s, l, g Measurements: number & unit: -2. 0 ml (volume) -14. 8 g (mass)

Chemical Properties • Describe how matter behaves in presence of other matter • Describe how matter changes into another kind of matter – Flammability – Corrosiveness – Combustibility

Properties of Copper • • Physical Prop Reddish brown Shiny Malleable Ductile Good Conductor Density = 8. 92 g/cm 3 MP = 1085 C BP = 2570 C Chemical Prop • Reacts to form green copper carbonate • Forms a deep blue solution with NH 3 • Forms copper nitrate (new substance) with HNO 3

Physical Change • The form or appearance of sample may change but the identity remains same – Cutting, crushing, grinding, tearing – Phase changes – Dissolving

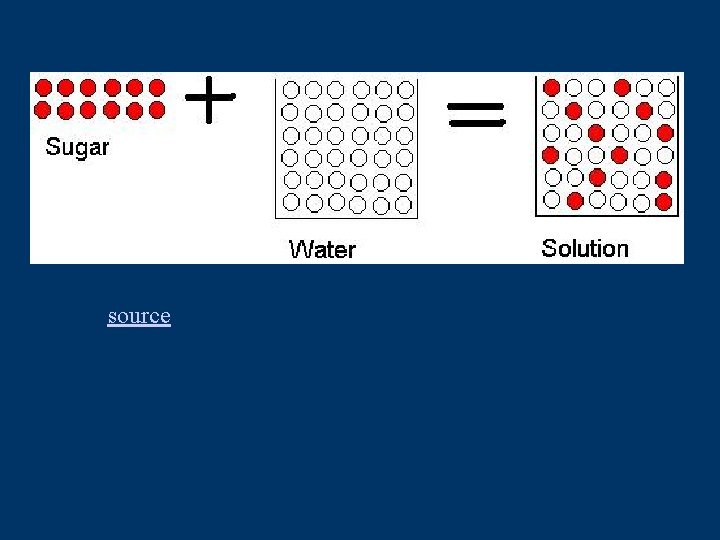
Dissolving • Dissolving is a physical change • Think of sugar in water • still have sugar – just spread out with water molecules in between • C 6 H 12 O 6(s) C 6 H 12 O 6(aq)

source

Phase Changes • Phase changes are physical changes • No new substance is created • chemical formula stays same Ex: ice melting: H 2 O(s) H 2 O(l) water boiling: H 2 O (l) H 2 O(g)

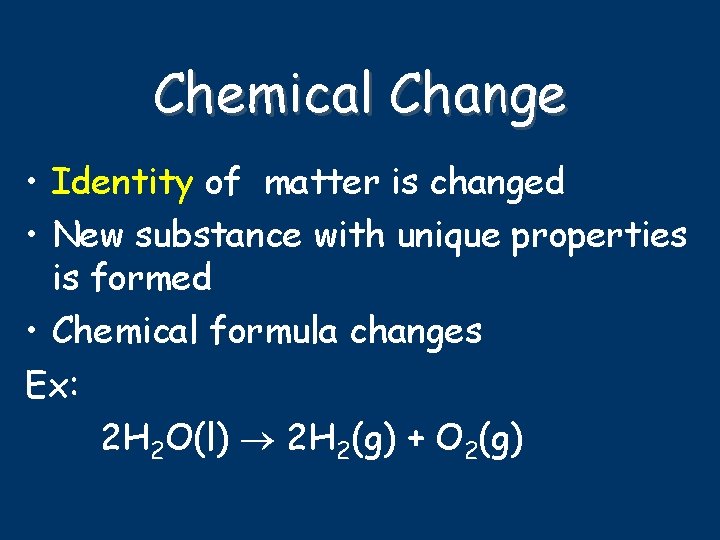
Chemical Change • Identity of matter is changed • New substance with unique properties is formed • Chemical formula changes Ex: 2 H 2 O(l) 2 H 2(g) + O 2(g)

Burning • Burning means reacting with oxygen • Burning is chemical change: • original substance is changed into new kind(s) of matter

What kinds of matter are there?

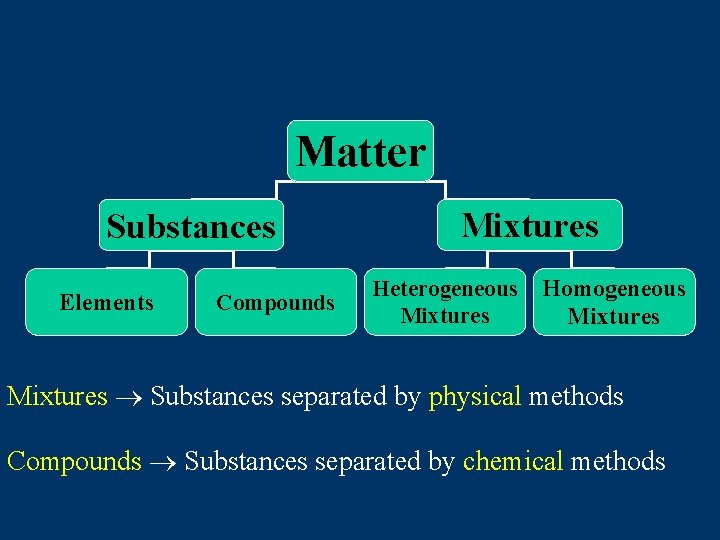
Matter Substances Elements Compounds Mixtures Heterogeneous Mixtures Homogeneous Mixtures Substances separated by physical methods Compounds Substances separated by chemical methods