What is matter Matter is anything that occupies




- Slides: 14



What is matter? Matter is anything that occupies space and has mass. Everything – everything: a cat, a leaf, a book, a raindrop, a flea, a pencil, even you! – on Earth is made up of matter. Matter has three forms, which are known as the three states of matter: o Solid o Liquid o Gas.

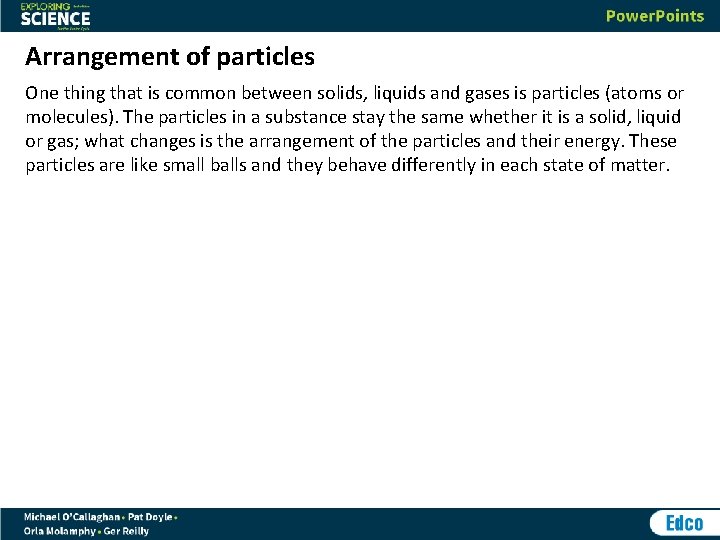
Arrangement of particles One thing that is common between solids, liquids and gases is particles (atoms or molecules). The particles in a substance stay the same whether it is a solid, liquid or gas; what changes is the arrangement of the particles and their energy. These particles are like small balls and they behave differently in each state of matter.

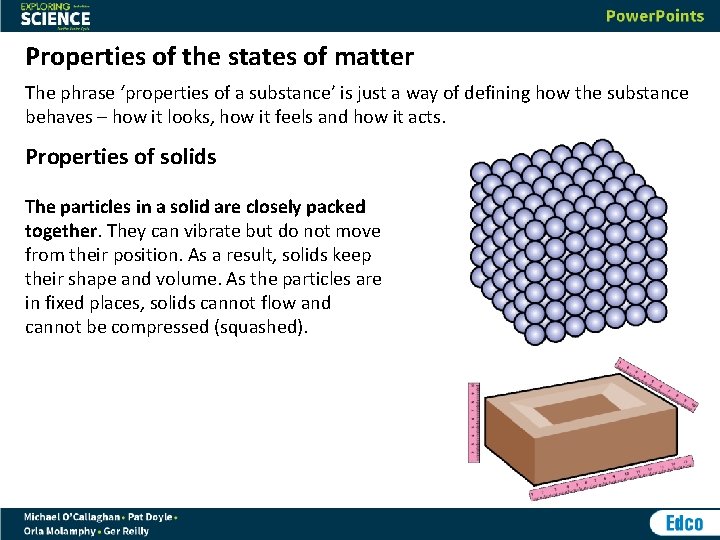
Properties of the states of matter The phrase ‘properties of a substance’ is just a way of defining how the substance behaves – how it looks, how it feels and how it acts. Properties of solids The particles in a solid are closely packed together. They can vibrate but do not move from their position. As a result, solids keep their shape and volume. As the particles are in fixed places, solids cannot flow and cannot be compressed (squashed).

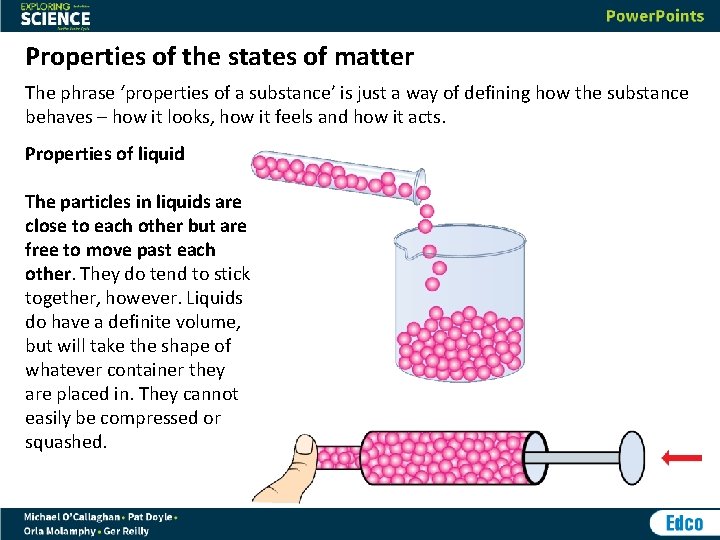
Properties of the states of matter The phrase ‘properties of a substance’ is just a way of defining how the substance behaves – how it looks, how it feels and how it acts. Properties of liquid The particles in liquids are close to each other but are free to move past each other. They do tend to stick together, however. Liquids do have a definite volume, but will take the shape of whatever container they are placed in. They cannot easily be compressed or squashed.

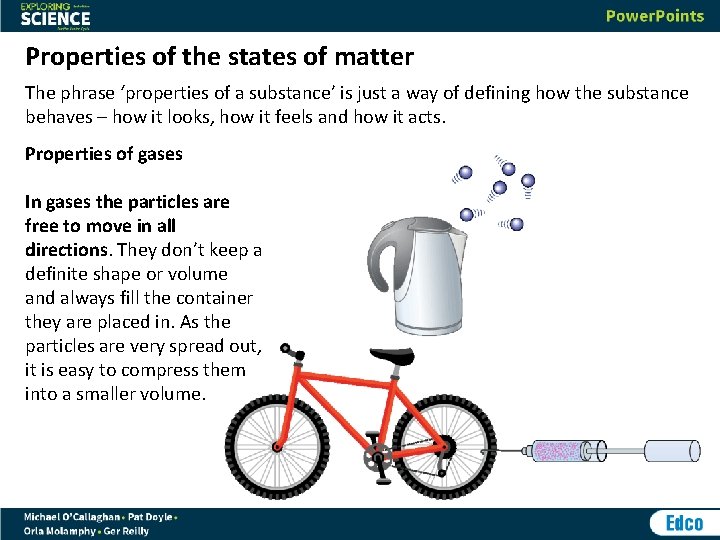
Properties of the states of matter The phrase ‘properties of a substance’ is just a way of defining how the substance behaves – how it looks, how it feels and how it acts. Properties of gases In gases the particles are free to move in all directions. They don’t keep a definite shape or volume and always fill the container they are placed in. As the particles are very spread out, it is easy to compress them into a smaller volume.

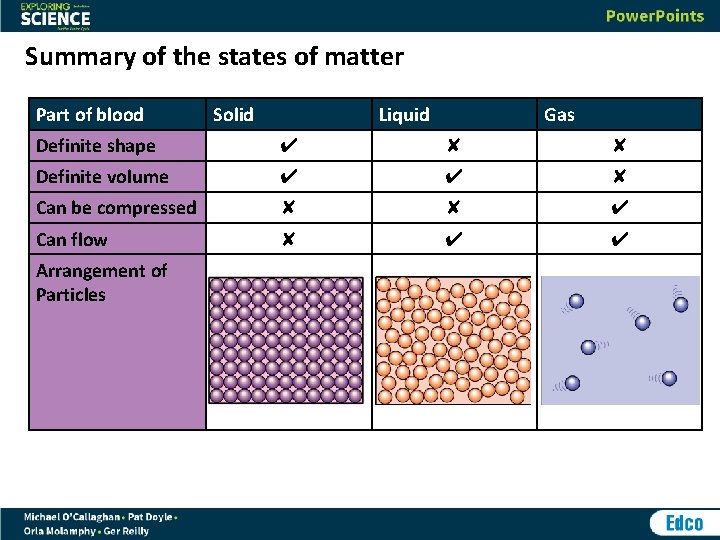
Summary of the states of matter Part of blood Solid Liquid Gas Definite shape ✔ ✘ ✘ Definite volume ✔ ✔ ✘ Can be compressed ✘ ✘ ✔ Can flow ✘ ✔ ✔ Arrangement of Particles

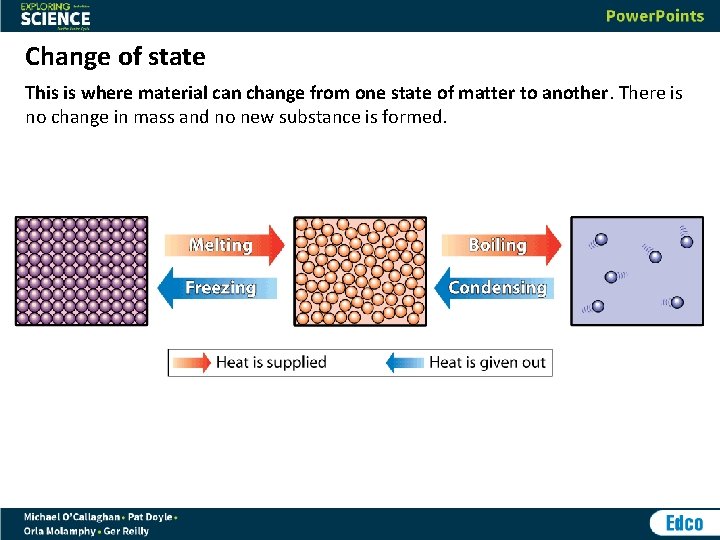
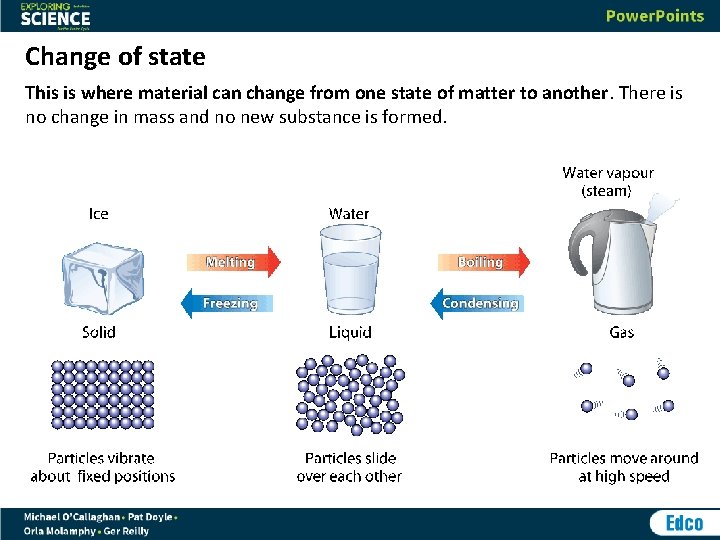
Change of state This is where material can change from one state of matter to another. There is no change in mass and no new substance is formed.

Change of state This is where material can change from one state of matter to another. There is no change in mass and no new substance is formed.

Change of state Melting takes place when a solid is heated (gains energy) and it changes into a liquid. Boiling takes place when a liquid is heated and it turns into a gas. Condensation is where cooling a gas (removing energy) causes it to change into a liquid. Freezing happens when a liquid is cooled and changes into a solid.

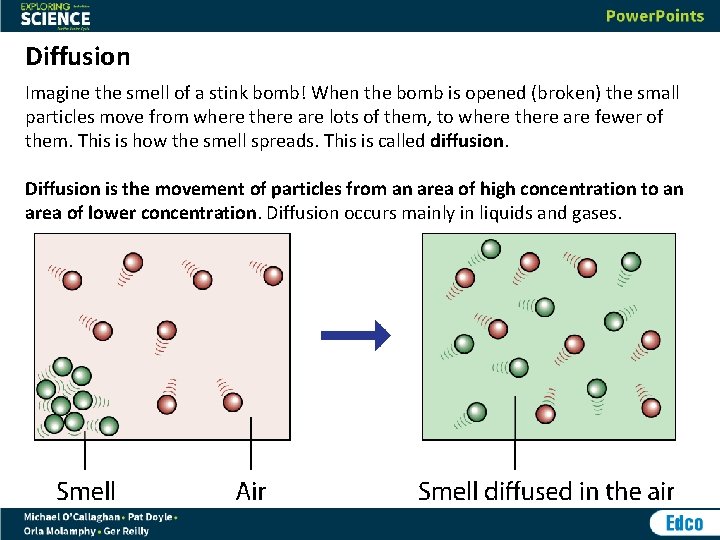
Diffusion Imagine the smell of a stink bomb! When the bomb is opened (broken) the small particles move from where there are lots of them, to where there are fewer of them. This is how the smell spreads. This is called diffusion. Diffusion is the movement of particles from an area of high concentration to an area of lower concentration. Diffusion occurs mainly in liquids and gases.

Fourth state of matter Plasma is the fourth state of matter. It is very similar to a gas – in fact, plasma is a gas that can carry an electrical charge. Plasma particles spread out and move around randomly, but unlike gas, plasma has the ability to conduct electricity.

Photo credits • Michael Philips • QBS
 Matter is anything that occupies space and has
Matter is anything that occupies space and has Anything which occupies space and has mass
Anything which occupies space and has mass Matter is anything that:
Matter is anything that: It is anything that has mass and occupies space
It is anything that has mass and occupies space It is anything that has mass and occupies space
It is anything that has mass and occupies space Tôn thất thuyết là ai
Tôn thất thuyết là ai Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Walmart thất bại ở nhật
Walmart thất bại ở nhật Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block xoang nhĩ ecg
Block xoang nhĩ ecg Tìm vết của đường thẳng
Tìm vết của đường thẳng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thể thơ truyền thống
Thể thơ truyền thống Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật
























