Molecules of Life Matter is anything that occupies








- Slides: 25

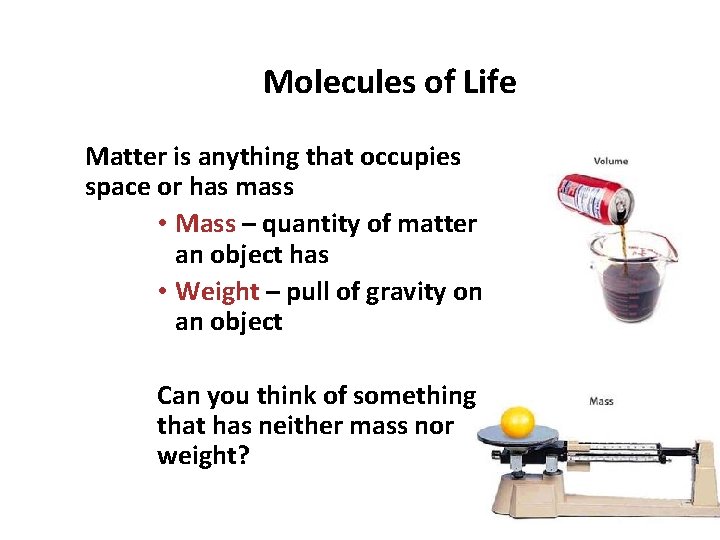
Molecules of Life Matter is anything that occupies space or has mass • Mass – quantity of matter an object has • Weight – pull of gravity on an object Can you think of something that has neither mass nor weight?

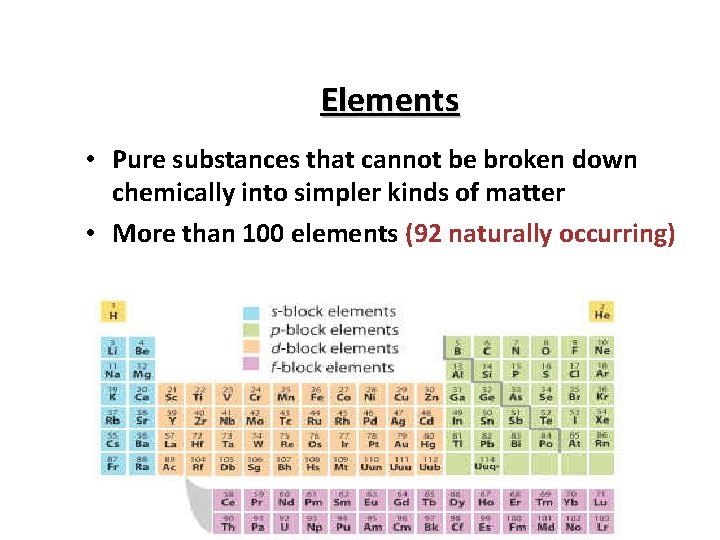
Elements • Pure substances that cannot be broken down chemically into simpler kinds of matter • More than 100 elements (92 naturally occurring)

Atoms • The simplest particle of an element that retains all the properties of that element • Properties of atoms determine the structure and properties of the matter they compose

The Nucleus • Central core • Consists of positive charged protons and neutral neutrons • Positively charged • Contains most of the mass of the atom

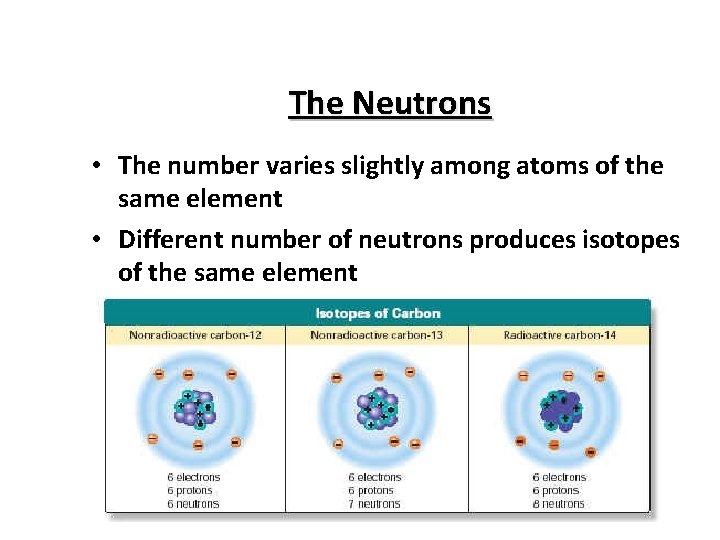
The Neutrons • The number varies slightly among atoms of the same element • Different number of neutrons produces isotopes of the same element

The Protons • All atoms of a given element have the same number of protons • Number of protons called the atomic number • Number of protons balanced by an equal number of negatively charged electrons

The Electrons • Negatively charged high energy particles with little or no mass • Travel at very high speeds at various distances (energy levels) from the nucleus • Are located around the nucleus

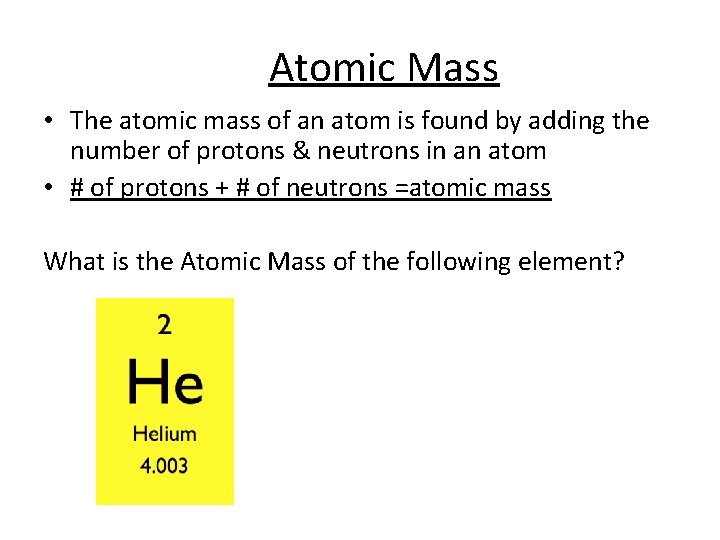
Atomic Mass • The atomic mass of an atom is found by adding the number of protons & neutrons in an atom • # of protons + # of neutrons =atomic mass What is the Atomic Mass of the following element?

90% of the mass of an organism is composed of 4 elements 1. 2. 3. 4. oxygen carbon hydrogen nitrogen

____ ATOMS MOLECULES _____ ORGANELLES ______

CELLS TISSUES ____________ Similar cells working together IMAGE SOURCES: see last slide

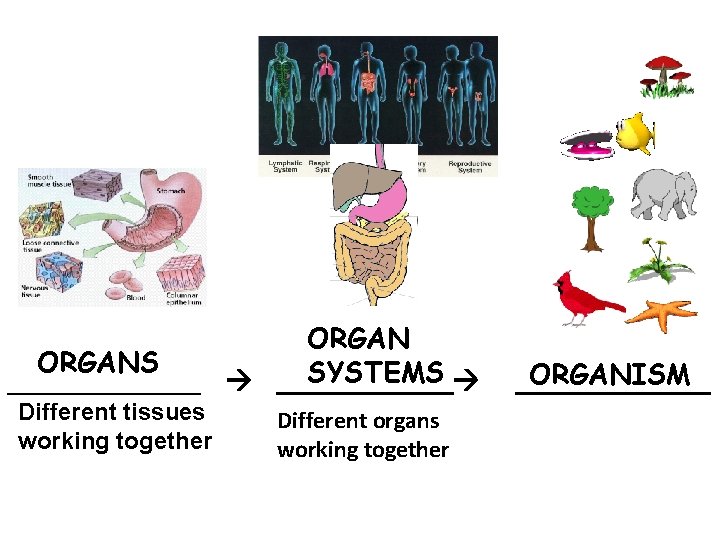
ORGANS SYSTEMS ______ Different tissues working together Different organs working together ORGANISM ______

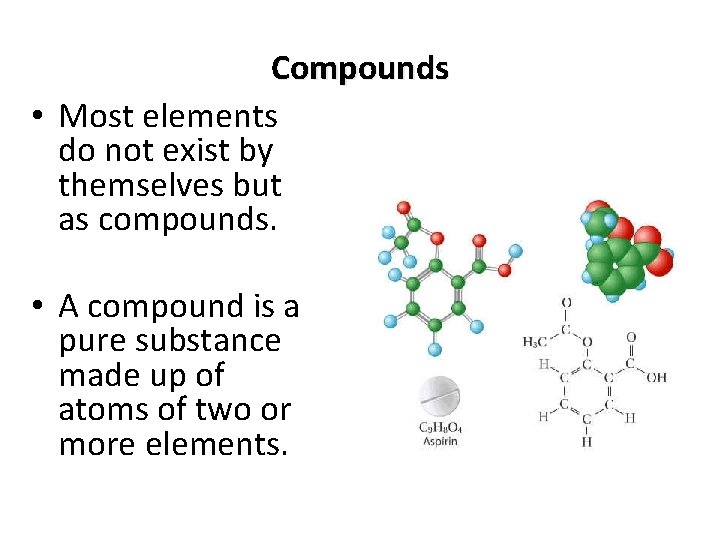
Compounds • Most elements do not exist by themselves but as compounds. • A compound is a pure substance made up of atoms of two or more elements.

Is H 20 an element or a compound? Why?

Carbon Compounds • Contain carbon atoms, an element with four valence electrons • The most common biomolecule or basic building blocks of cells.

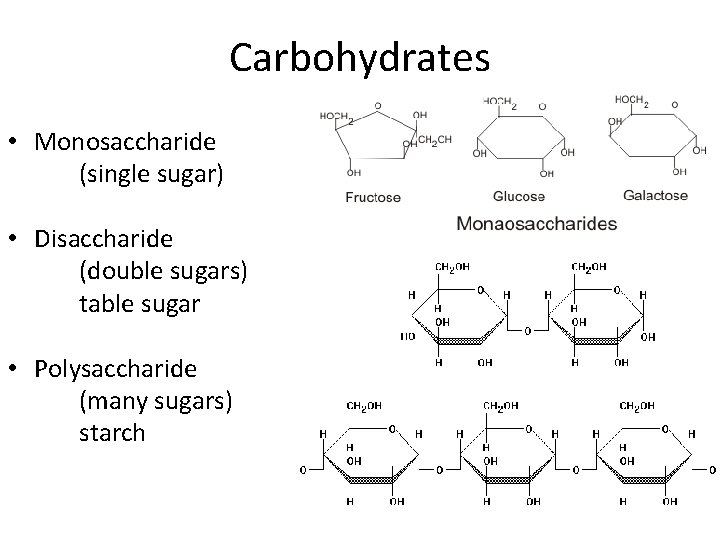
Carbohydrates • One of the four main carbon molecules • Made of carbon, hydrogen, and oxygen in a 1: 2: 1 ratio

Carbohydrates • Monosaccharide (single sugar) • Disaccharide (double sugars) table sugar • Polysaccharide (many sugars) starch

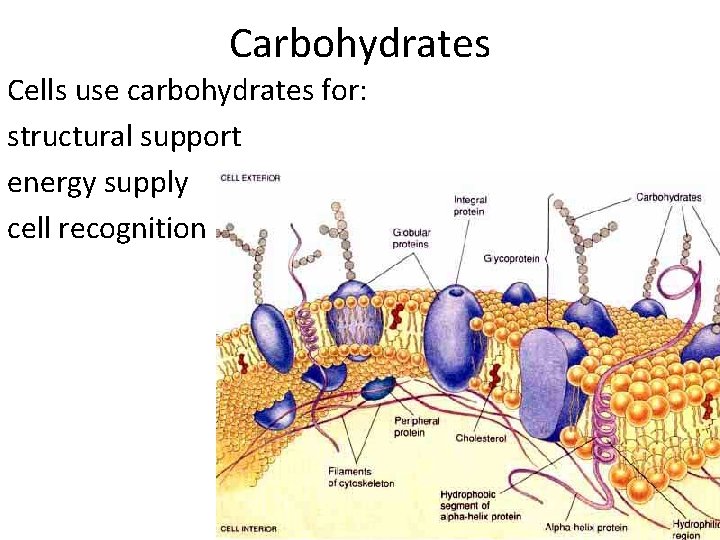
Carbohydrates Cells use carbohydrates for: structural support energy supply cell recognition

Lipids • Consist of chains of carbon atoms bonded to each other and to hydrogen atoms.

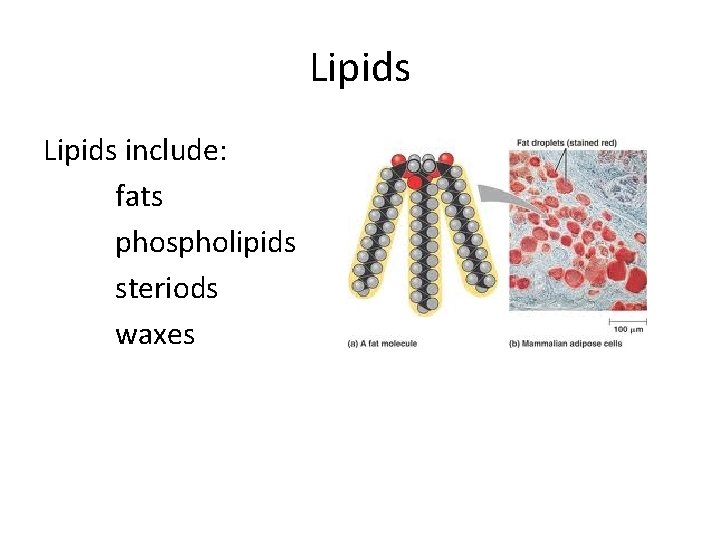
Lipids include: fats phospholipids steriods waxes

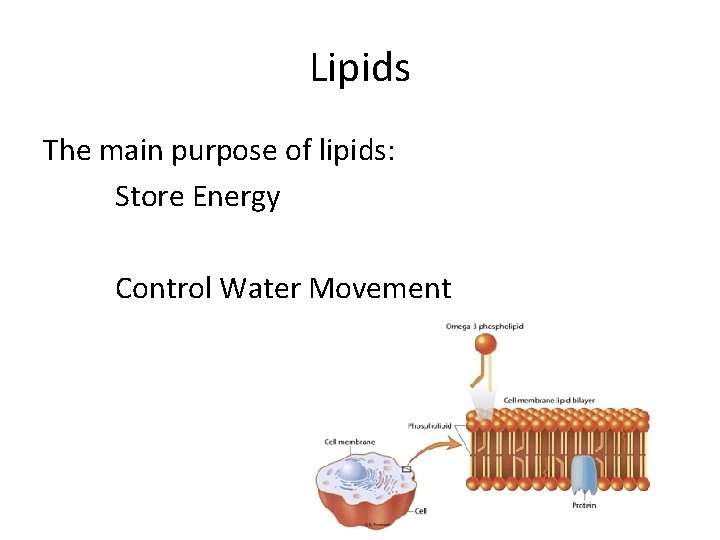
Lipids The main purpose of lipids: Store Energy Control Water Movement

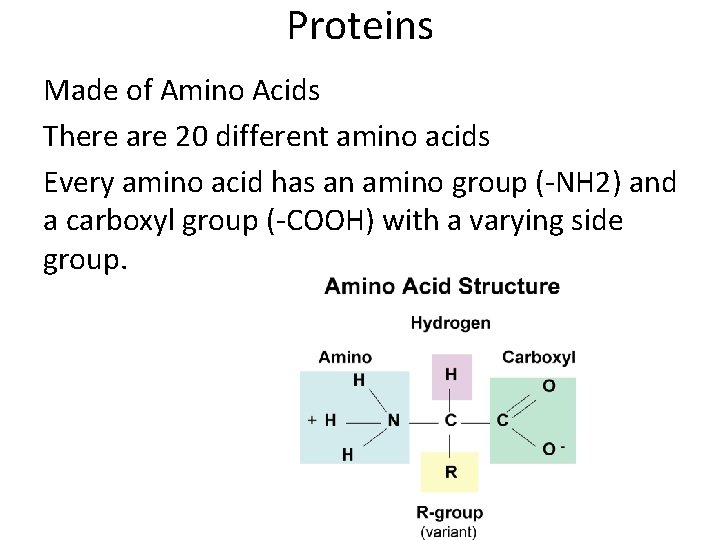
Proteins Made of Amino Acids There are 20 different amino acids Every amino acid has an amino group (-NH 2) and a carboxyl group (-COOH) with a varying side group.

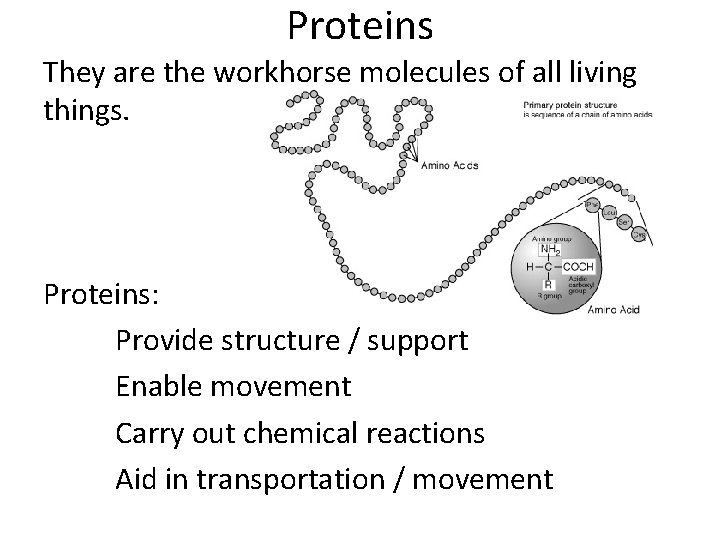
Proteins They are the workhorse molecules of all living things. Proteins: Provide structure / support Enable movement Carry out chemical reactions Aid in transportation / movement

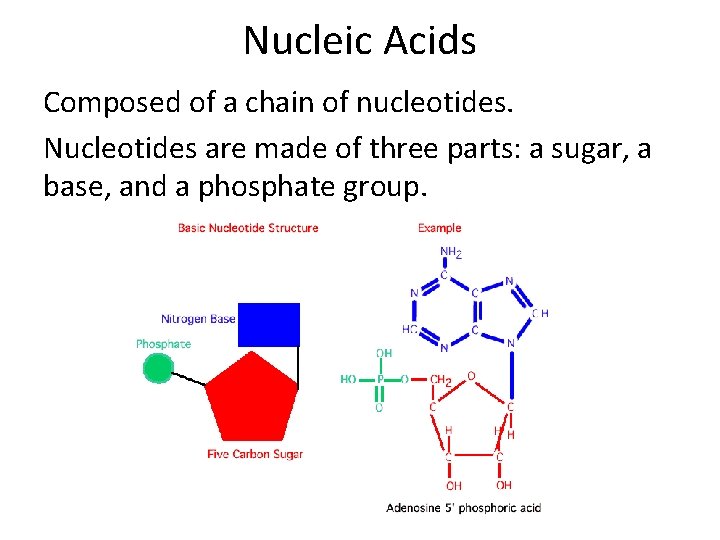
Nucleic Acids Composed of a chain of nucleotides. Nucleotides are made of three parts: a sugar, a base, and a phosphate group.

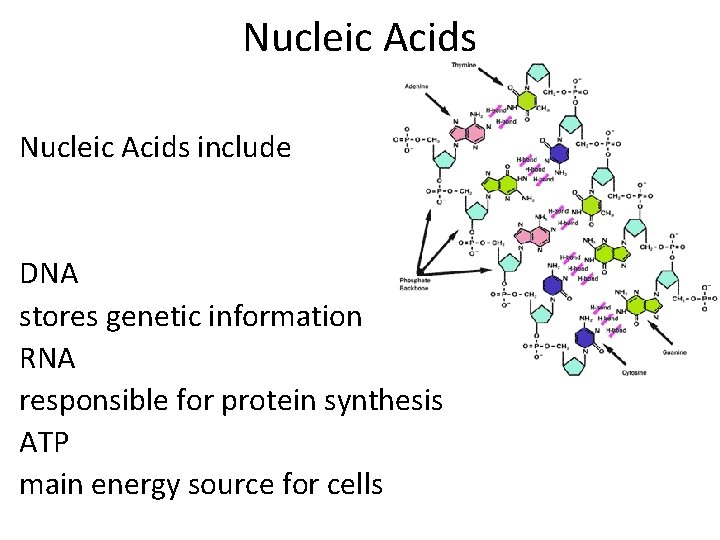
Nucleic Acids include DNA stores genetic information RNA responsible for protein synthesis ATP main energy source for cells
 What is occupies space and has mass
What is occupies space and has mass Matter is anything that occupies space and has mass
Matter is anything that occupies space and has mass Matter is anything that
Matter is anything that It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter has mass and occupies space
Matter has mass and occupies space Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Phân độ lown
Phân độ lown Block nhĩ thất độ 3
Block nhĩ thất độ 3 Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt What is mass
What is mass Matter anything that
Matter anything that Matter is anything that has and takes up
Matter is anything that has and takes up Matter is defined as anything that
Matter is defined as anything that Anything that has mass and volume
Anything that has mass and volume All matter has and takes up
All matter has and takes up Anything that takes up space and has mass is
Anything that takes up space and has mass is Is matter anything that has mass
Is matter anything that has mass Matter anything that
Matter anything that Matter is anything that...
Matter is anything that...