States of Matter Matter is anything that occupies














- Slides: 43

States of Matter

Matter is anything that occupies space and has mass. Three States of Matter: • Solids • Liquids • Gases

Three states of matter At room temperature most substances exist in one of three physical states. solid liquid gas

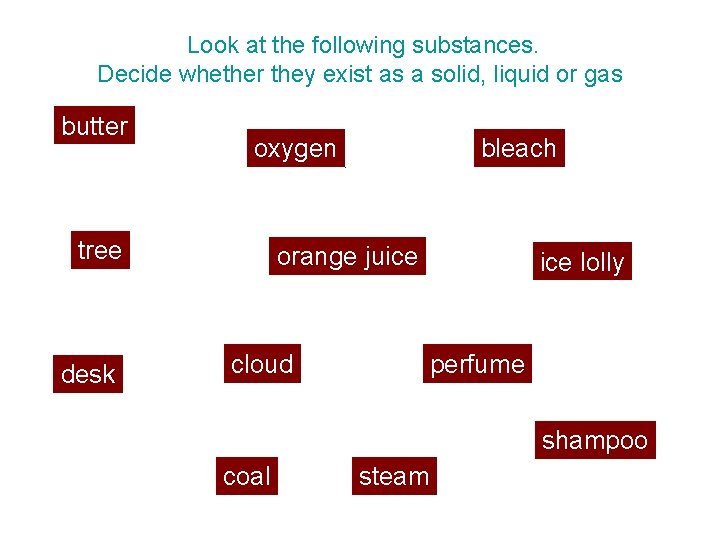
Look at the following substances. Decide whether they exist as a solid, liquid or gas. butter oxygen tree desk bleach orange juice cloud ice lolly perfume shampoo coal steam

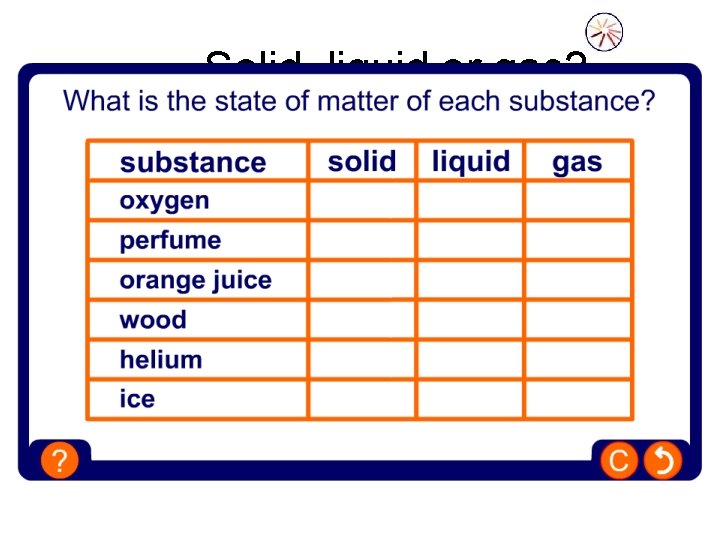
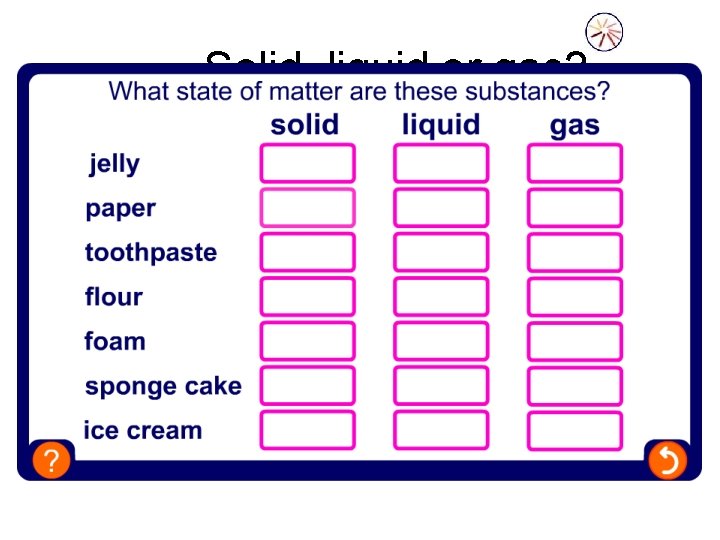
Solid, liquid or gas?

Solid, liquid or gas?

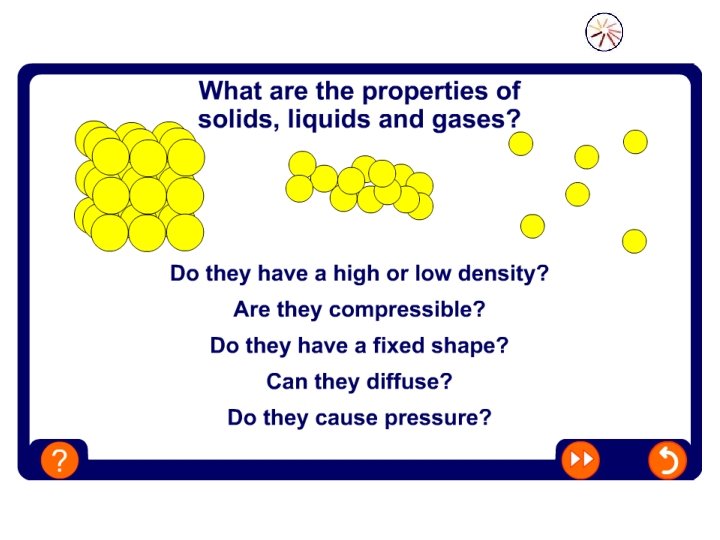
What you should learn about today. . . • About the particle model for the states of matter • What are the characteristics of the states of matter • What diffusion is

Can you remember what the three states of matter are? 1. Solids 2. Liquids 3. Gases All matter is made up of particles These particles are so tiny that we would need a really powerful microscope to see them

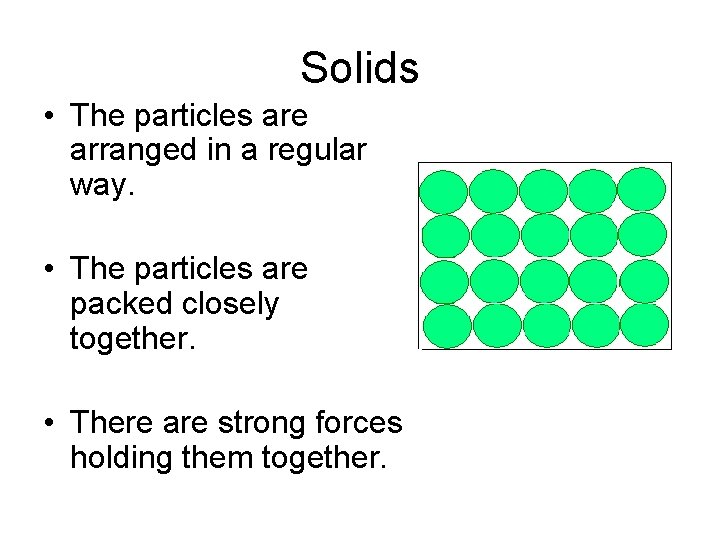
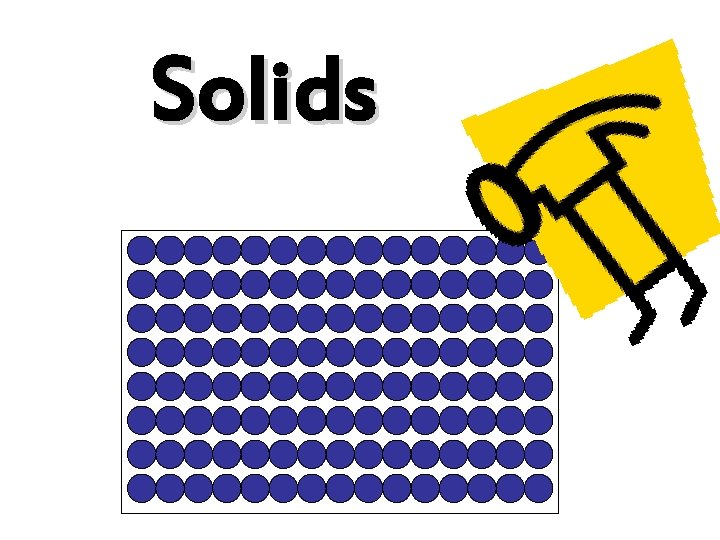
Solids • The particles are arranged in a regular way. • The particles are packed closely together. • There are strong forces holding them together.

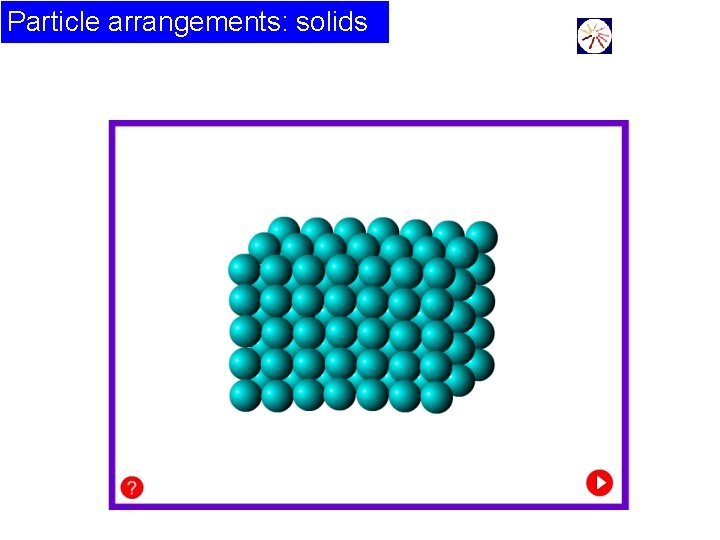
Particle arrangements: solids This animation shows a 2 -D view of the motion of the atoms in a 3 -D solid.

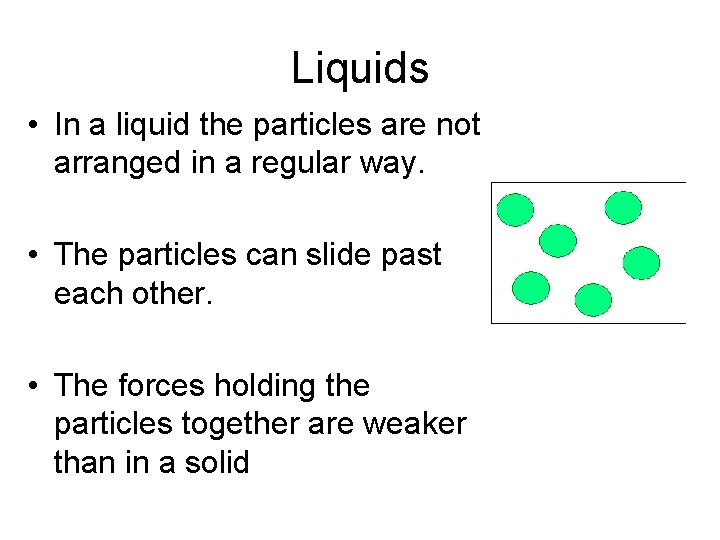
Liquids • In a liquid the particles are not arranged in a regular way. • The particles can slide past each other. • The forces holding the particles together are weaker than in a solid

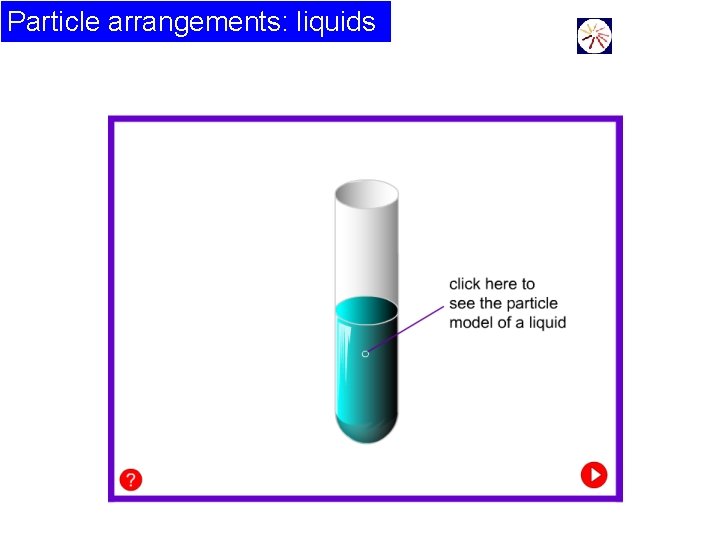
Particle arrangements: liquids This animation shows a 2 -D view of the motion of the atoms in a liquid. There is no order.

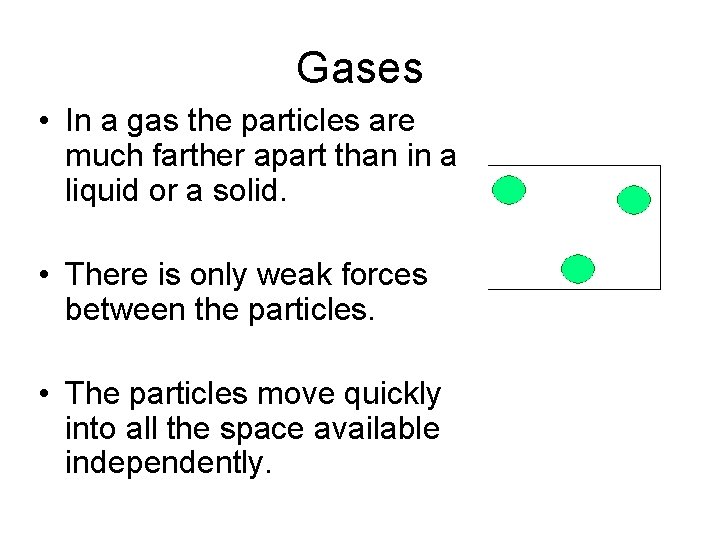
Gases • In a gas the particles are much farther apart than in a liquid or a solid. • There is only weak forces between the particles. • The particles move quickly into all the space available independently.

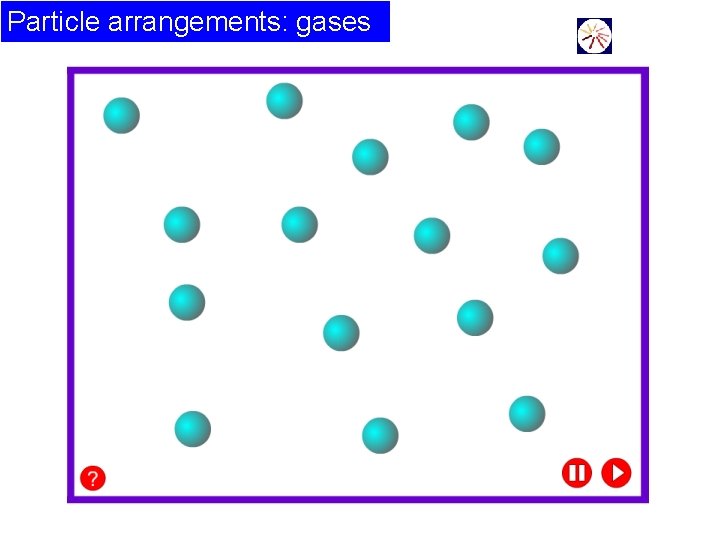
Particle arrangements: gases

Solids

The Particles are packed tightly together. The bonds between them are strong. They have a definite shape They have a definite volume They cannot be compressed (squashed) Cannot flow


The Particles are loosely connected. The bonds between them are weak, they can move easily! They have no definite shape They have a fixed volume They cannot be compressed( squashed) They can flow

Gases

The Particles are not joined at all. They can move freely with speed. They have no definite shape (They expand to fill any space) They have no definite volume They can flow and spread out They can be compressed (SQUASHED)


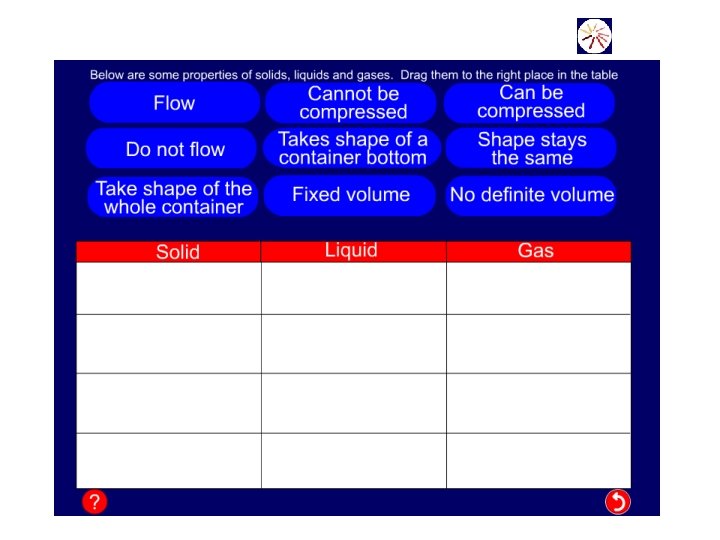
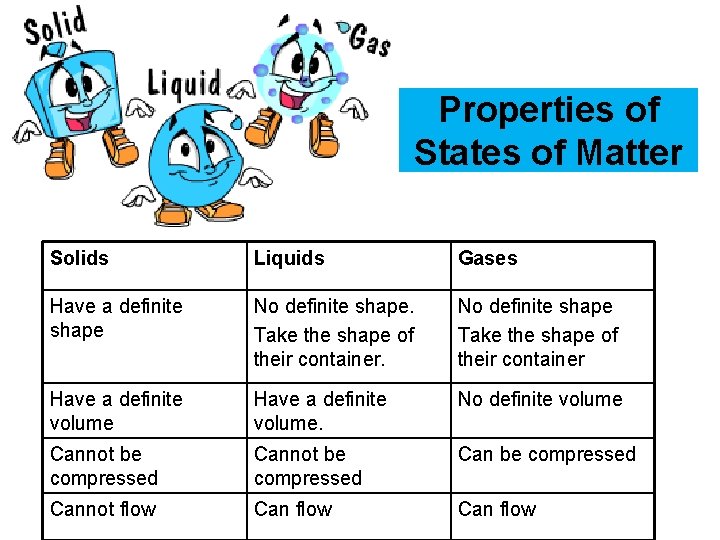
Properties of States of Matter Solids Liquids Gases Have a definite shape No definite shape. Take the shape of their container. No definite shape Take the shape of their container Have a definite volume. No definite volume Cannot be compressed Cannot flow Can flow

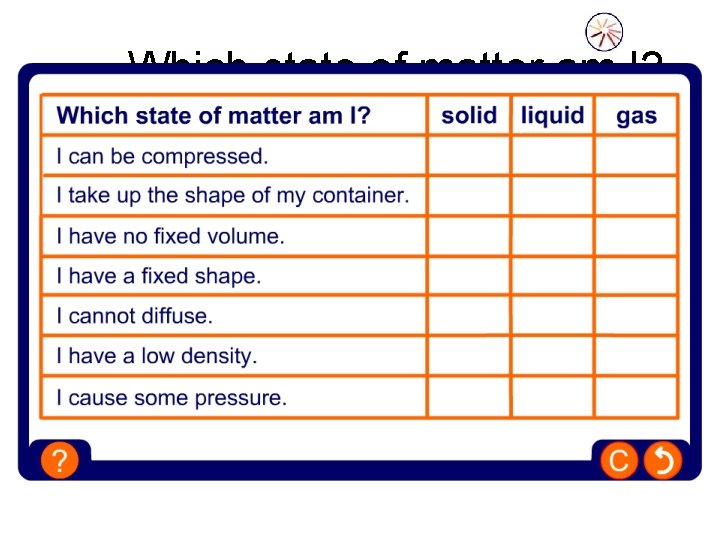
Which state of matter am I?


How do smells spread out? Where is the smell coming from and how does it spread out?

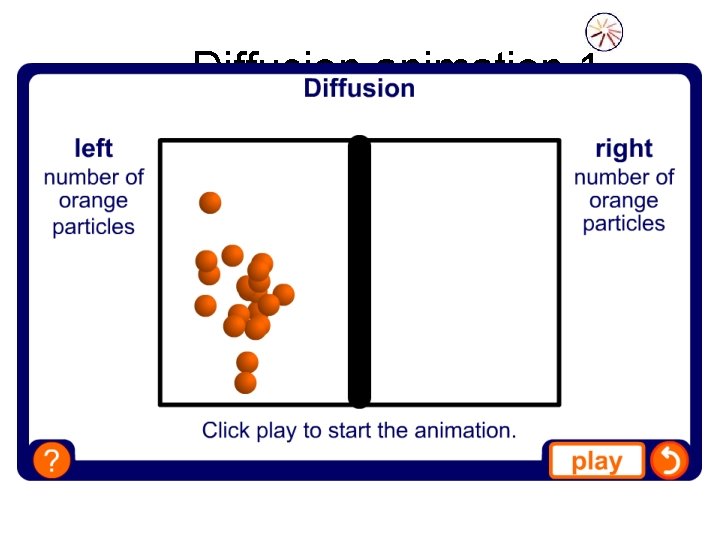
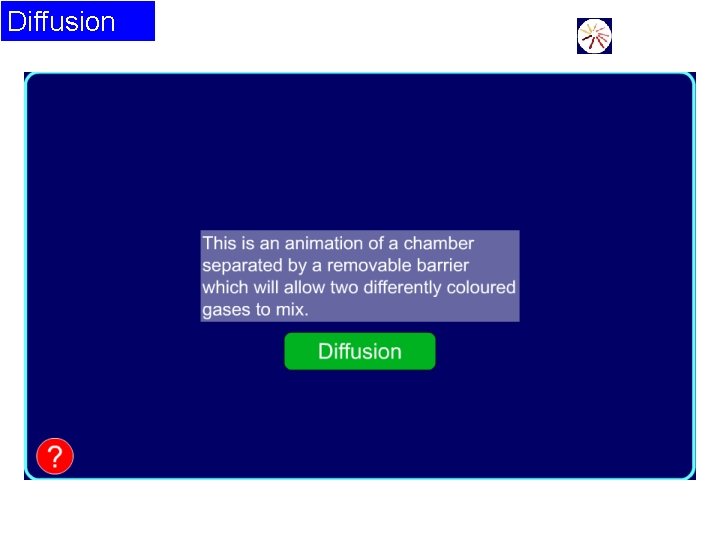
Diffusion • Diffusion is the way in which particles in gases and liquids spread out the space in which they are placed. • For example, the smell of after-shave or perfume diffuses across a room. Insects communicate by smells that diffuse through the air.

Diffusion animation 1

Diffusion in a liquid Activity 2 • Drop a piece of potassium permanganate into the bottom of the beaker of water with a straw. In your notes copy: • Draw a picture of the experiment. • Write about what we did (the method), and say what happened (the result). .

Diffusion • Can you explain why…. Diffusion occurs both in liquids and gases but hardly at all in solids? Diffusion happens more quickly for gases than for liquids.

Diffusion

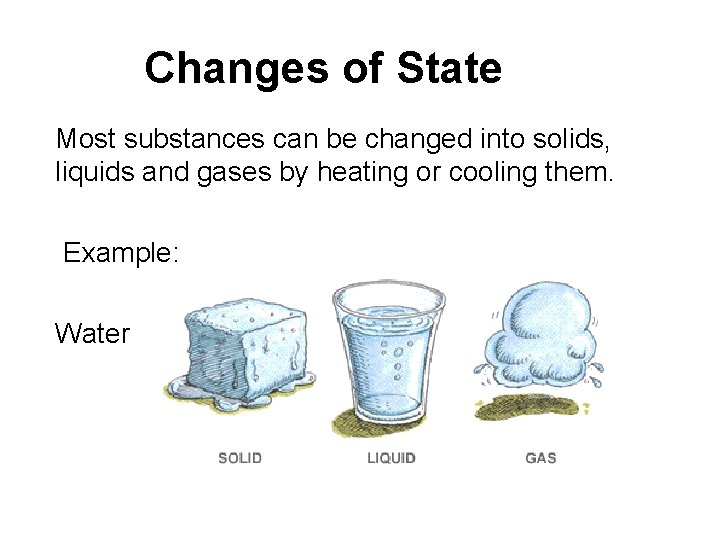
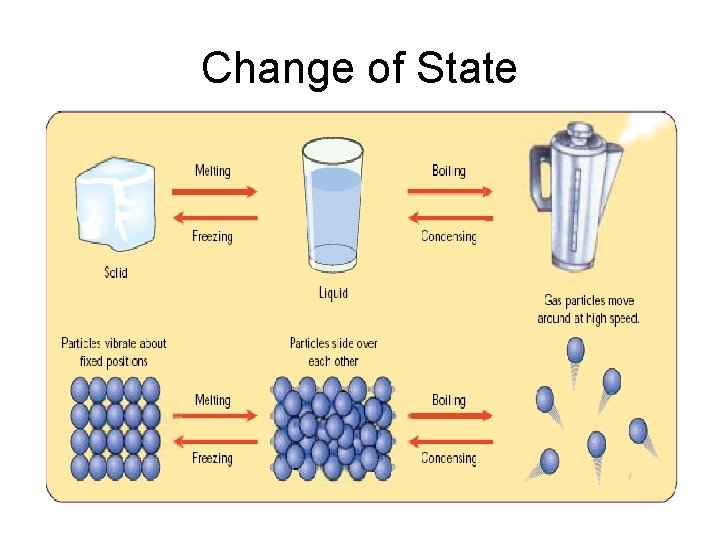
Changes of State Most substances can be changed into solids, liquids and gases by heating or cooling them. Example: Water

Matter can change states Many materials can change into each of the different states. For example, we know that water can be a solid (ICE), liquid (WATER) and gas (STEAM).

Melting is the changing of a solid to a liquid

Icecaps in the Artic are melting because of global warming. If we can’t stop this the polar bears won’t have a place to live

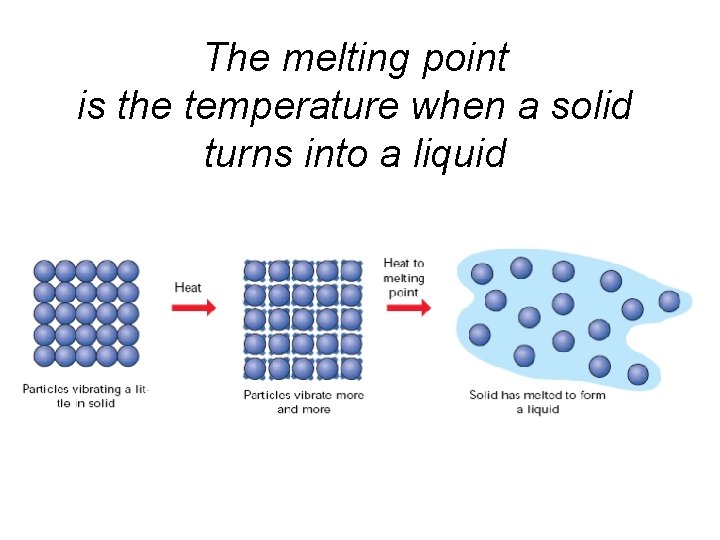
The melting point is the temperature when a solid turns into a liquid

Have you noticed that puddles of rain dry up quickly on a sunny day? Evaporation is the changing of a liquid to a gas The heat from the sun gives some of the particles at the surface of the liquid extra energy and they escape from the liquid and go into the air!

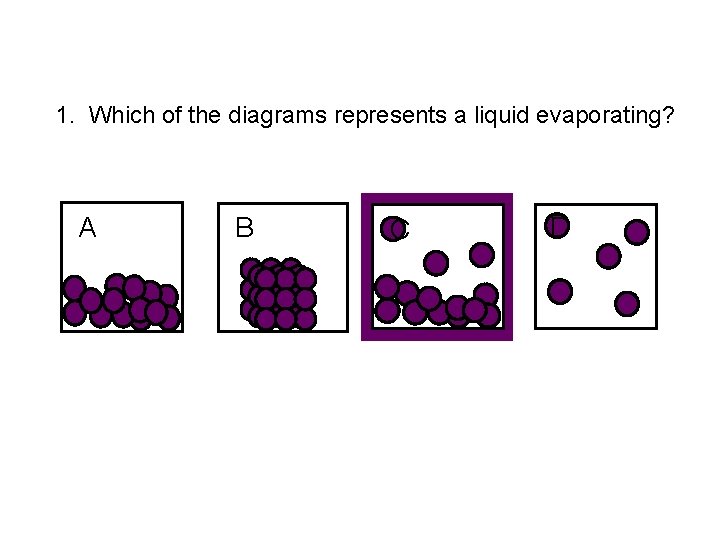
1. Which of the diagrams represents a liquid evaporating? A B C D

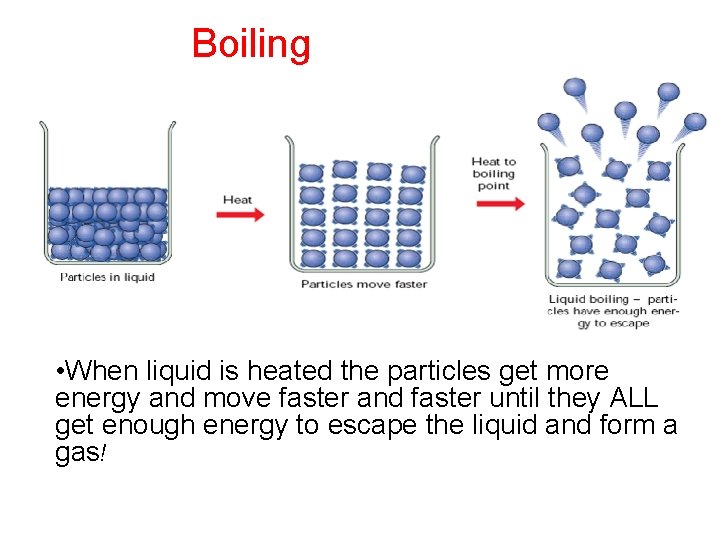
Boiling • The boiling point is the temperature when a liquid turns into a gas

Boiling • When liquid is heated the particles get more energy and move faster and faster until they ALL get enough energy to escape the liquid and form a gas!

On a cold morning have you ever noticed that the inside of the car window was covered in moisture? Condensation is the changing of a gas to a liquid

Freezing Is the changing of a liquid to a solid When liquid candle wax cools down it freezes and becomes a solid…

In some places it gets so cold that the lakes freeze over …

Change of State
 Matter is anything that takes up space and has mass
Matter is anything that takes up space and has mass Matter is anything that occupies space
Matter is anything that occupies space Matter is anything that occupies space and has
Matter is anything that occupies space and has It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter has mass and occupies space
Matter has mass and occupies space Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thể thơ truyền thống
Thể thơ truyền thống Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Tôn thất thuyết là ai
Tôn thất thuyết là ai Phân độ lown
Phân độ lown Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block nhĩ thất cấp 1
Block nhĩ thất cấp 1 Matter anything that
Matter anything that Matter is anything that
Matter is anything that Anything that has volume or mass is
Anything that has volume or mass is Matter vs weight
Matter vs weight Matter is anything that:
Matter is anything that: Matter is anything that has mass and volume
Matter is anything that has mass and volume Matter anything that
Matter anything that Matter anything that
Matter anything that Anything that has mass
Anything that has mass Dmv formula
Dmv formula Matter is anything that:
Matter is anything that: All matter has and takes up
All matter has and takes up Matter anything that
Matter anything that Matter is anything that...
Matter is anything that... Anything that has mass and takes up space is
Anything that has mass and takes up space is Anything that has mass and takes up space
Anything that has mass and takes up space No matter anything
No matter anything Matter is anything that has
Matter is anything that has Matter anything that
Matter anything that Defintion of matter
Defintion of matter Matter is defined as anything that
Matter is defined as anything that 11 free states
11 free states Southern states vs northern states
Southern states vs northern states Tyranny
Tyranny Chapter 12 states of matter study guide
Chapter 12 states of matter study guide Whats the study of matter and energy
Whats the study of matter and energy Examples of gas
Examples of gas Particle theory of matter examples
Particle theory of matter examples Thermal energy vs heat
Thermal energy vs heat