STATES OF MATTER MATTER Matter is anything that





























- Slides: 39

STATES OF MATTER

MATTER Matter is anything that takes up space and has mass. Matter does not have to be visible. Air is matter.

States of Matter • • Three states of matter common on Earth: Solids Liquids Gases States of matter in the Universe Plasma Bose-Einstein Condensate 1995 Fermionic Condensate 2004

The Fifth State of Matter • • Bose-Einstein Condensation in a gas: a new form of matter at the coldest temperatures in the universe. . . It is the ability to slow down light. Atoms move faster than photons. • • Predicted 1924. . . Created 1995 • A. Einstein S. Bose

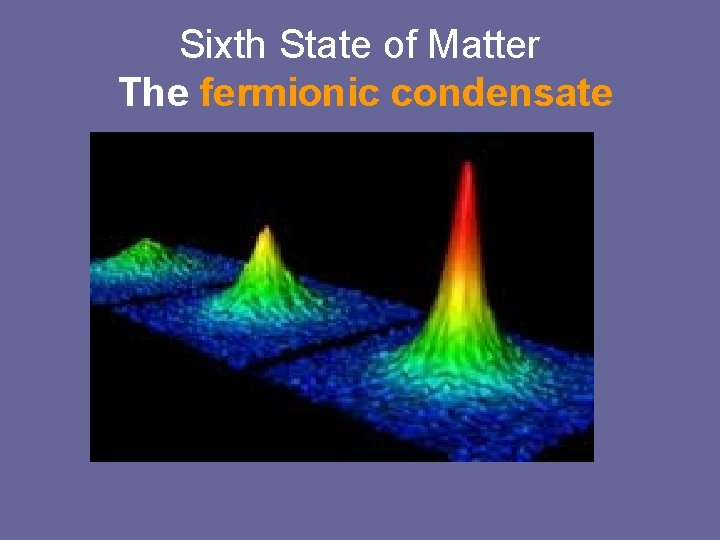
The Sixth State of Matter • Scientists have created a new form of matter, which they say could lead to new ways of transmitting electricity and communication to the Moon and other planets. • The fermionic condensate is a cloud of cold potassium (P -40) atoms forced into a state where they behave strangely. It is considered a super cooled, strange superparticle and a superfluid.

Sixth State of Matter The fermionic condensate

Solid • Solid is matter with a define shape and volume. • Particles that make up all types of matter are in constant motion.

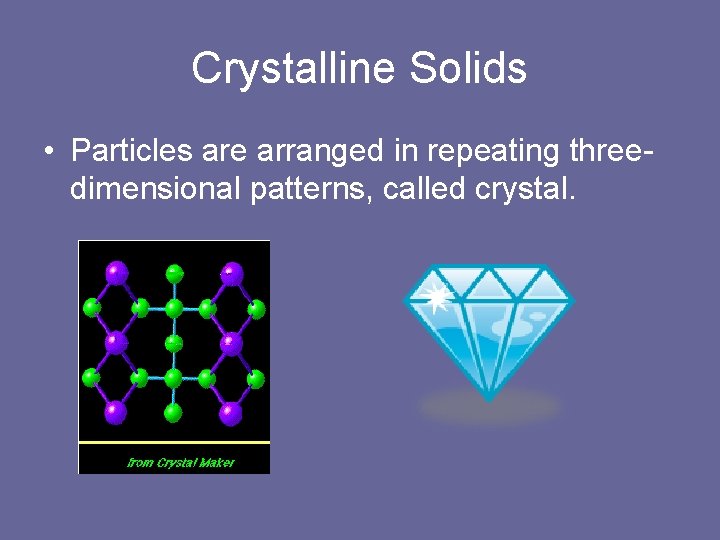
Crystalline Solids • Particles are arranged in repeating threedimensional patterns, called crystal.

Amorphous Solids • Solids with large particles arranged randomly. • Example: plastic, rubber.

Surface Tension • Uneven forces acting on the particles of the surface of the liquid. • The surface of the liquid acts as a thin film were stretched across its surface. Ex: floating an insect on the surface of water.

Gases • Matter does not have a definite shape or volume. Vapor Matter that exist in the gas state but is generally a liquid or solid at room temperature. Ex: water is a liquid. water vapor is a gas.

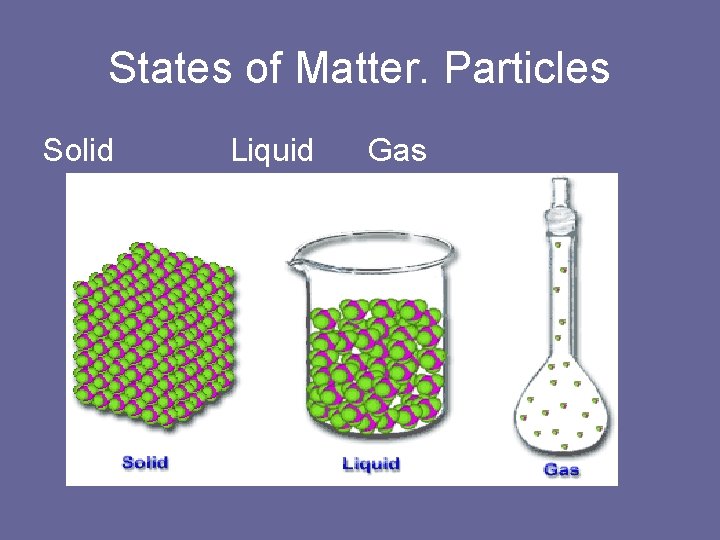
States of Matter. Particles Solid Liquid Gas

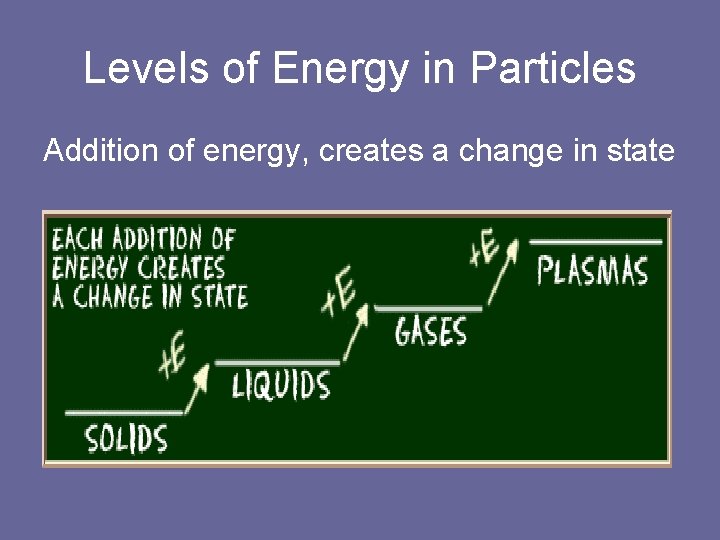
Levels of Energy in Particles Addition of energy, creates a change in state

Changes of State of Matter • Kinetic Energy is the total energy of motion. • Particles of matter are at constant motion. • The amount of motion depends of the kinetic energy of the particles. • Particles moving slowly have less kinetic energy. ( solids) • Particles moving faster have more kinetic energy. ( gases)

Temperature • The average kinetic energy of the particles that make up a substance.

Heat • The movement of thermal energy from a substance at a higher temperature to one at a lower temperature is called heat.

Specific Heat • The specific heat of a substance is the amount of heat required to raise the temperature of 1 Kg. of a substance in 1 Celsius degree.

Matter. - Materials that have a low specific heat • Metals • Aluminum, Gold Sand

Materials that have high specific heat • Water

Melting • The change from a solid to the liquid state.

Freezing • The change from the liquid state to the solid state.

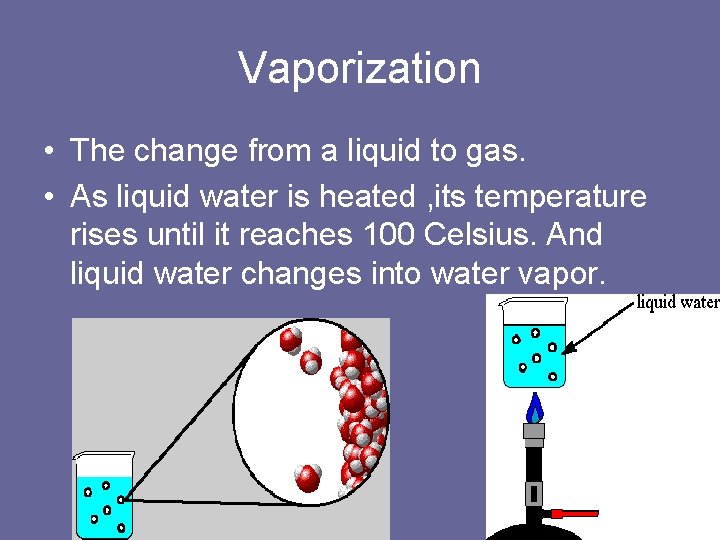
Vaporization • The change from a liquid to gas. • As liquid water is heated , its temperature rises until it reaches 100 Celsius. And liquid water changes into water vapor.

Evaporation • The conversion of water from a liquid into a gas. • Water is transferred from the surface to the atmosphere through evaporation, the process by which water changes from a liquid to a gas.

Evaporation

Condensation • It is the opposite process of vaporization. • A gas condenses to a liquid. • The decrease in energy changes the arrangements of the particles.

Sublimation • This is a drawing of what the surface of a comet might look like. This is a picture of dry ice (frozen CO 2) sublimating.

Sublimation • Sublimation of an element or compound is a transition from the solid to gas phase so rapidly that the liquid phase cannot be observed. Sublimation is a phase of transition where solid gain enough energy to transform in gas.

Deposition • The process of a gas changing directly into a solid is called deposition or desublimation. • For example, water vapor in sub-freezing air can transform into ice • without going through • the liquid phase, which is • how snow and frost are formed.

Viscosity • Some liquids flow more easily than others. • A liquid resistance to flow is known as viscosity. • Slower the liquid flow, higher the viscosity. • High viscosity pure honey.

Behavior of Fluids • Pressure is the force exerted on a surface divided by the total area over which the force is exerted. • • P= F/ A Force is measure in Newtons N. Area is measured in m 2 ( square meter) N/m 2 the unit is called Pascal ( Pa)

Pressure • Steam machine. Pumps water out of mines. ( Thomas Savery 17 th century )

Pressure • Crushing a can is applying pressure.

Pascal Principle • Atmospheric pressure at the sea level is 101. 3 KPa ( kilopascals). This means that air exerts a force about 101, 000 N ( Newtons).

Atmospheric Pressure • Changes with altitude. • Altitude is the height above the sea level. • As altitude increases, atmospheric pressure decreases.


Buoyant Force/Archimede’s Principle • The difference in pressure results in an upward force on an object immerse in a fluid. • The buoyant force is equal to the weight of the object.

Buoyancy • Sink or float

Archimedes’Principle • Density is mass divided by volume • D= m/v • Understanding density can help you predict whether an object will float or sink.

Pascal’s principle • When a force is applied to a confined fluid, an increase in pressure is transmitted equally to all parts of the fluid. • This relationship is known as Pascal’s Principle.
 Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Tôn thất thuyết là ai
Tôn thất thuyết là ai Phân độ lown
Phân độ lown Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block xoang nhĩ là gì
Block xoang nhĩ là gì Is anything that has mass and takes up space.
Is anything that has mass and takes up space. Matter is anything that:
Matter is anything that: Anything that has mass and takes up space
Anything that has mass and takes up space No matter anything
No matter anything Benjamin cummings
Benjamin cummings Matter is anything that...
Matter is anything that... This is anything with mass that occupies space.
This is anything with mass that occupies space. What is matter
What is matter Matter is anything that occupies space and has -----------
Matter is anything that occupies space and has ----------- Melting point defintion
Melting point defintion Matter is anything that has mass and
Matter is anything that has mass and Matter anything that
Matter anything that Defintion of matter
Defintion of matter Matter is anything with mass and volume
Matter is anything with mass and volume Matter is defined as anything that
Matter is defined as anything that No matter anything
No matter anything All matter has and takes up
All matter has and takes up Matter is anything that has
Matter is anything that has Matter anything that
Matter anything that Matter is anything that
Matter is anything that Something that takes up space
Something that takes up space Matter is anything that
Matter is anything that Matter anything that
Matter anything that Matter anything that
Matter anything that 11 free states
11 free states Southern states vs northern states
Southern states vs northern states Checks and balances dbq
Checks and balances dbq States of matter jeopardy
States of matter jeopardy Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Interconversion of states of matter
Interconversion of states of matter