Environmental Systems Chapter 2 Matter anything that occupies















- Slides: 17

Environmental Systems Chapter 2

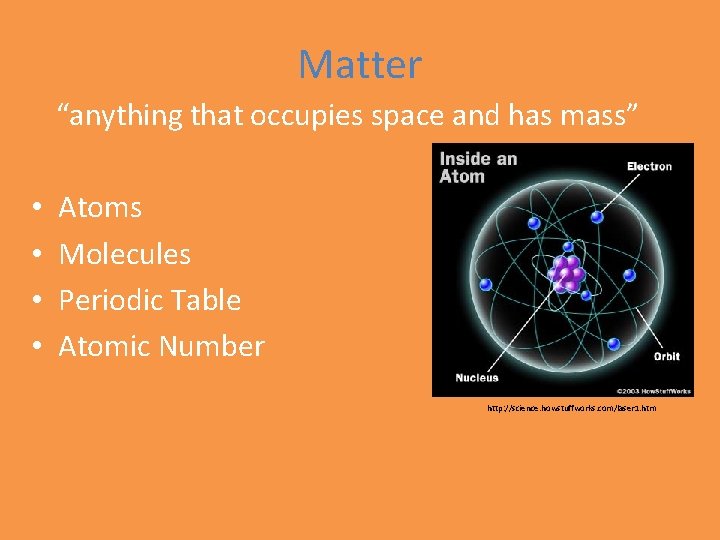
Matter “anything that occupies space and has mass” • • Atoms Molecules Periodic Table Atomic Number http: //science. howstuffworks. com/laser 1. htm

• No atoms are ever destroyed or created, but the bonds may change between them a. law of conservation of matter - “ matter cannot be created or destroyed; it can only change form”

Radioactivity • Unstable isotopes are radioactive • Radioactive decay a. spontaneous release of material from nucleus ex) Uranium-235 (235 U) • Half-life a. measurement of radioactive decay

Chemical Bonds • Covalent Bonds (sharing of e-) a. CH 4 (methane) • Ionic Bonds (transfer of e-) a. Na. Cl (salt) • Hydrogen Bonds (unequal sharing of e-) a. weak b. H 2 O

Properties of Water • • Surface tension Capillary action Boiling and freezing point Acts as a solvent http: //environment. nationalgeographic. com/environment/photos/freshwater-insects/

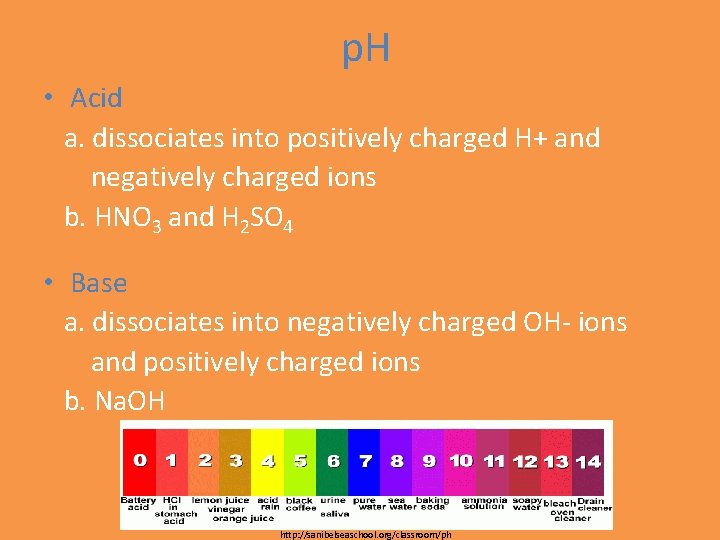
p. H • Acid a. dissociates into positively charged H+ and negatively charged ions b. HNO 3 and H 2 SO 4 • Base a. dissociates into negatively charged OH- ions and positively charged ions b. Na. OH http: //sanibelseaschool. org/classroom/ph

Organic Molecules • Organic v. Inorganic Compounds • Examples of Organic Compounds a. Carbohydrates b. Lipids c. Proteins d. Nucleic Acids

Energy • “ability to do work, or transfer heat” • Majority on Earth derives from the Sun

Forms of Energy • Joule: basic unit of energy (J) • Energy and Power a. energy-ability to do work power-rate at which work is done therefore, energy = power X time power = energy / time

• Kinetic and Potential Energy a. Potential-stored energy not yet released ex) water behind a dam b. Kinetic-energy of motion ex) electricity created from water captured by dam

Energy Laws • 1 st Law of Thermodynamics a. “energy is neither created nor destroyed” b. ex) Dams • 2 nd Law of Thermodynamics a. “when energy is transformed, the quantity of energy remains the same but its ability to do work diminishes”

b. energy efficiency c. energy quality - high v. low quality energy d. entropy e. global circulation patterns powered by Sun

Systems • Open System a. exchange of matter or energy across system boundaries ex) ocean • Closed System a. matter and energy exchange across the boundaries does not happen ex) underground cave system

• Input a. additions to a system • Output a. losses from the system

Steady State • “inputs equal outputs so that the system is not changing over time” • Allows us to know if the amount of a resource or pollutant is increasing, decreasing or staying the same

Feedbacks • Positive Feedback Loop a. amplifies change b. births in regards to human population • Negative Feedback Loop a. responds to a change by returning to its original state to reach homeostasis b. thermostat