Ch 10 11 Gases II The Gas Laws

- Slides: 14

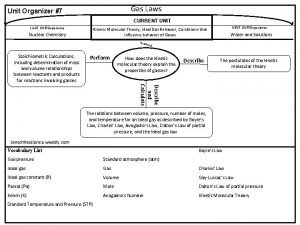
Ch. 10 & 11 - Gases II. The Gas Laws (p. 313 -322)

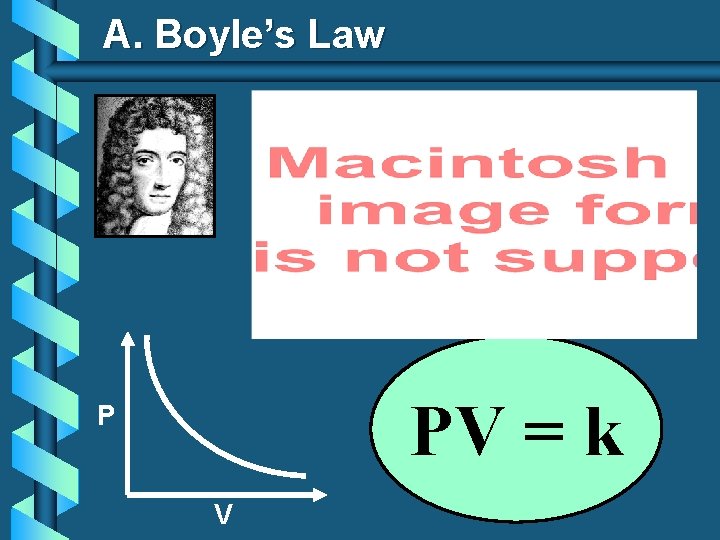
A. Boyle’s Law PV = k P V

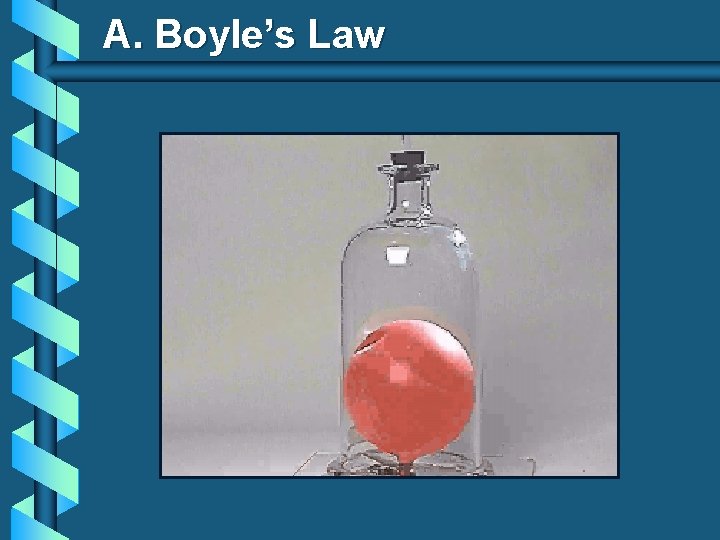
A. Boyle’s Law b The pressure and volume of a gas are inversely related • at constant mass & temp PV = k P V

A. Boyle’s Law

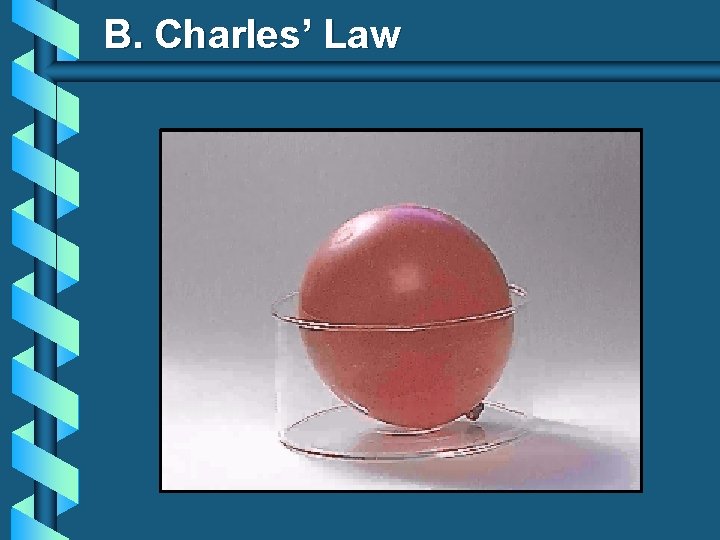
B. Charles’ Law V T

B. Charles’ Law b The volume and absolute temperature (K) of a gas are directly related • at constant mass & pressure V T

B. Charles’ Law

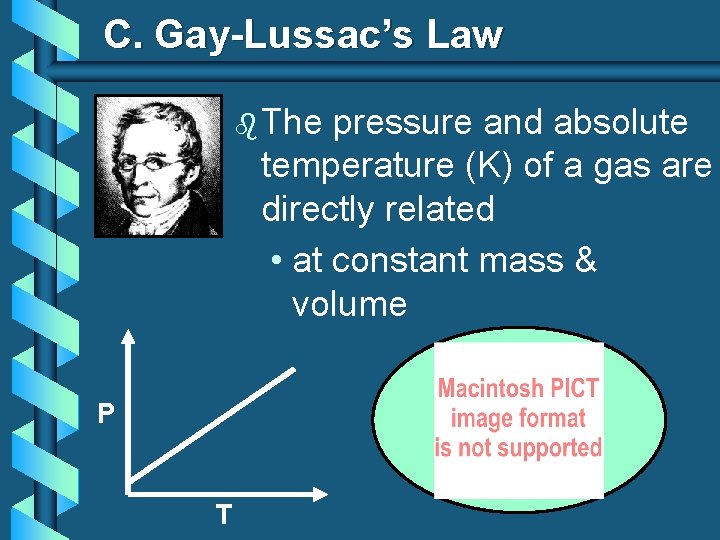
C. Gay-Lussac’s Law P T

C. Gay-Lussac’s Law b The pressure and absolute temperature (K) of a gas are directly related • at constant mass & volume P T

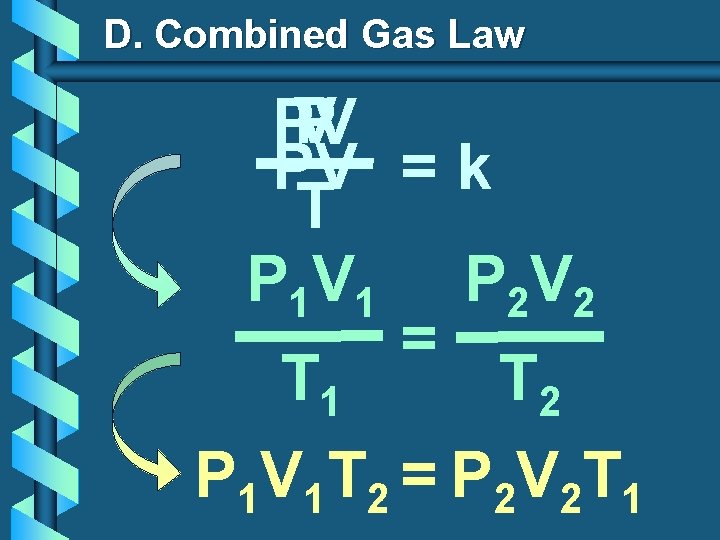
D. Combined Gas Law P V PV PV = k T P 1 V 1 P 2 V 2 = T 1 T 2 P 1 V 1 T 2 = P 2 V 2 T 1

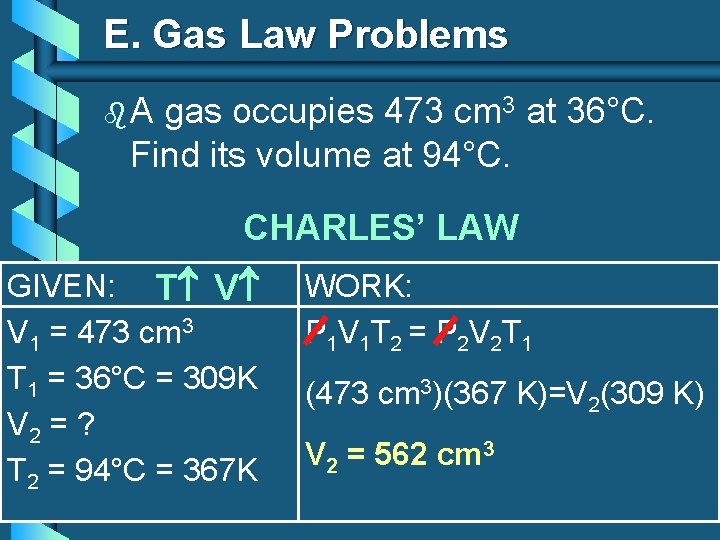
E. Gas Law Problems b. A gas occupies 473 cm 3 at 36°C. Find its volume at 94°C. CHARLES’ LAW GIVEN: T V V 1 = 473 cm 3 T 1 = 36°C = 309 K V 2 = ? T 2 = 94°C = 367 K WORK: P 1 V 1 T 2 = P 2 V 2 T 1 (473 cm 3)(367 K)=V 2(309 K) V 2 = 562 cm 3

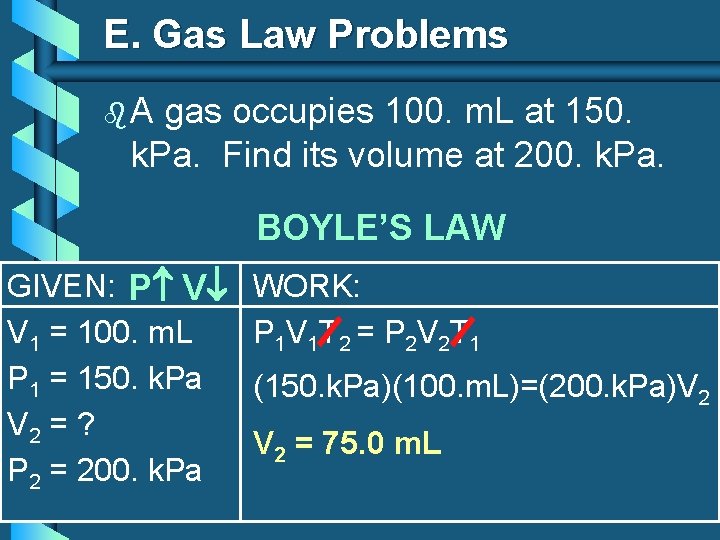
E. Gas Law Problems b. A gas occupies 100. m. L at 150. k. Pa. Find its volume at 200. k. Pa. BOYLE’S LAW GIVEN: P V V 1 = 100. m. L P 1 = 150. k. Pa V 2 = ? P 2 = 200. k. Pa WORK: P 1 V 1 T 2 = P 2 V 2 T 1 (150. k. Pa)(100. m. L)=(200. k. Pa)V 2 = 75. 0 m. L

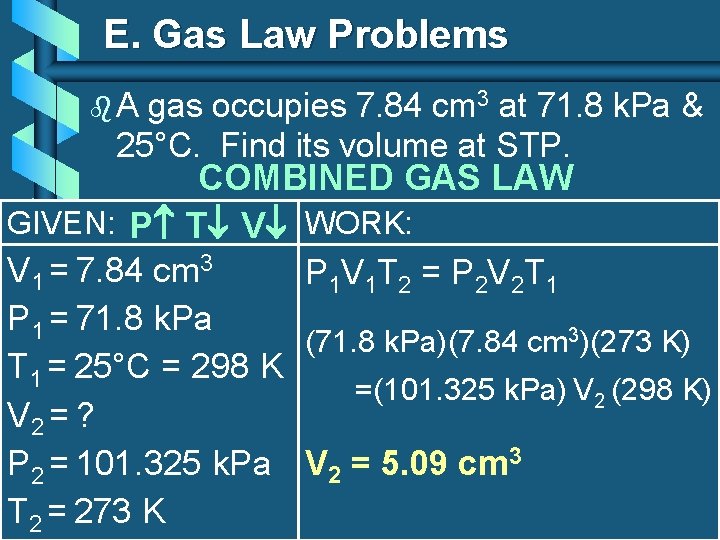
E. Gas Law Problems b. A gas occupies 7. 84 cm 3 at 71. 8 k. Pa & 25°C. Find its volume at STP. COMBINED GAS LAW GIVEN: P T V WORK: V 1 = 7. 84 cm 3 P 1 V 1 T 2 = P 2 V 2 T 1 P 1 = 71. 8 k. Pa (71. 8 k. Pa)(7. 84 cm 3)(273 K) T 1 = 25°C = 298 K =(101. 325 k. Pa) V 2 (298 K) V 2 = ? P 2 = 101. 325 k. Pa V 2 = 5. 09 cm 3 T 2 = 273 K

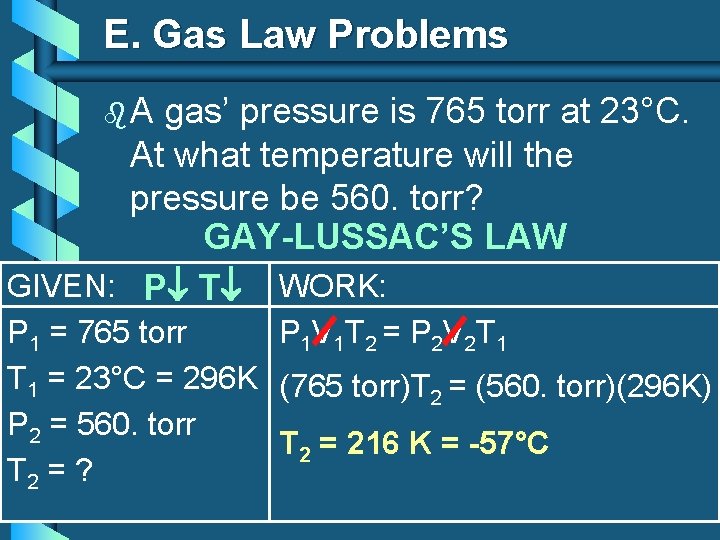
E. Gas Law Problems b. A gas’ pressure is 765 torr at 23°C. At what temperature will the pressure be 560. torr? GAY-LUSSAC’S LAW GIVEN: P T WORK: P 1 = 765 torr P 1 V 1 T 2 = P 2 V 2 T 1 = 23°C = 296 K (765 torr)T 2 = (560. torr)(296 K) P 2 = 560. torr T 2 = 216 K = -57°C T 2 = ?
 Charles de secondat
Charles de secondat Different gas laws
Different gas laws Avogadro's law formula
Avogadro's law formula Gas law graphic organizer
Gas law graphic organizer Boyle's law variables
Boyle's law variables Gas law formula
Gas law formula All the gas laws
All the gas laws Boyle's law
Boyle's law Section 13.2 the combined gas law and avogadro's principle
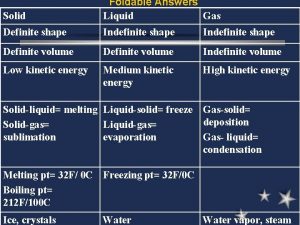
Section 13.2 the combined gas law and avogadro's principle Indefinite shape
Indefinite shape Charles law
Charles law Gas laws hot air balloon
Gas laws hot air balloon A gas occupies 473 cm3 at 36°c. find its volume at 94°c
A gas occupies 473 cm3 at 36°c. find its volume at 94°c Empirical gas law
Empirical gas law Combined gas law formula
Combined gas law formula