Ch 12 Gases I Physical Properties A Ideal


- Slides: 21

Ch. 12 - Gases I. Physical Properties

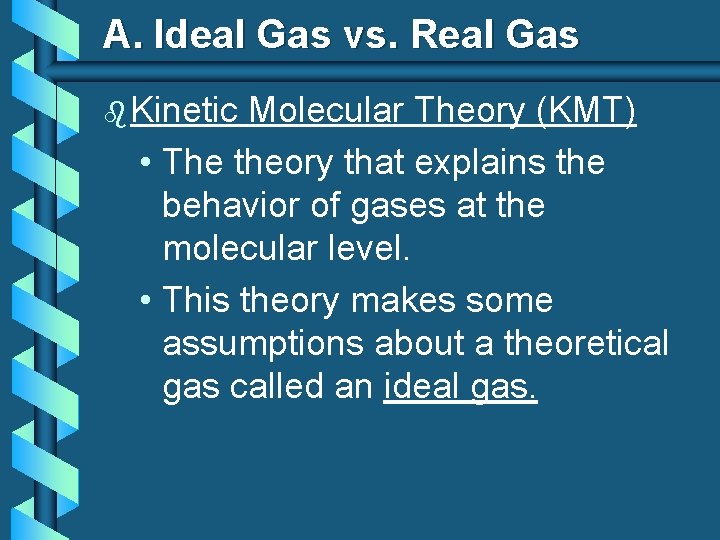
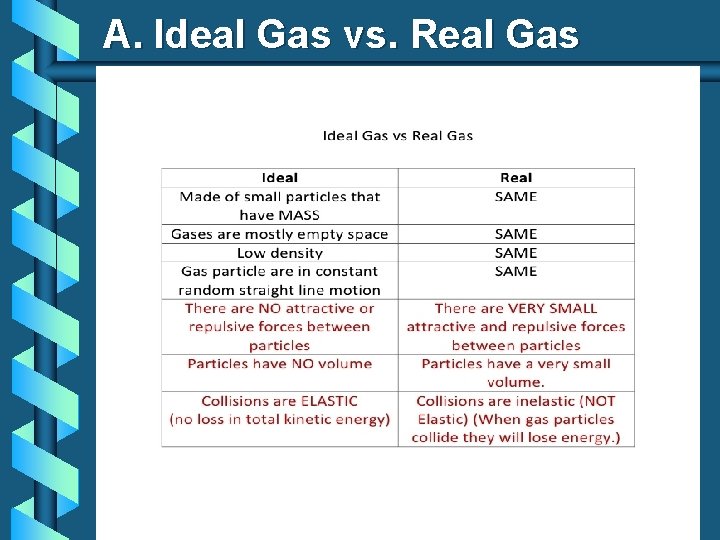
A. Ideal Gas vs. Real Gas b Kinetic Molecular Theory (KMT) • The theory that explains the behavior of gases at the molecular level. • This theory makes some assumptions about a theoretical gas called an ideal gas.

A. Ideal Gas vs. Real Gas

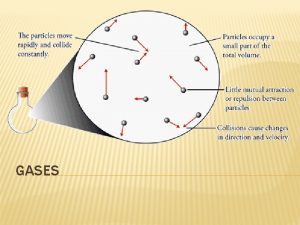
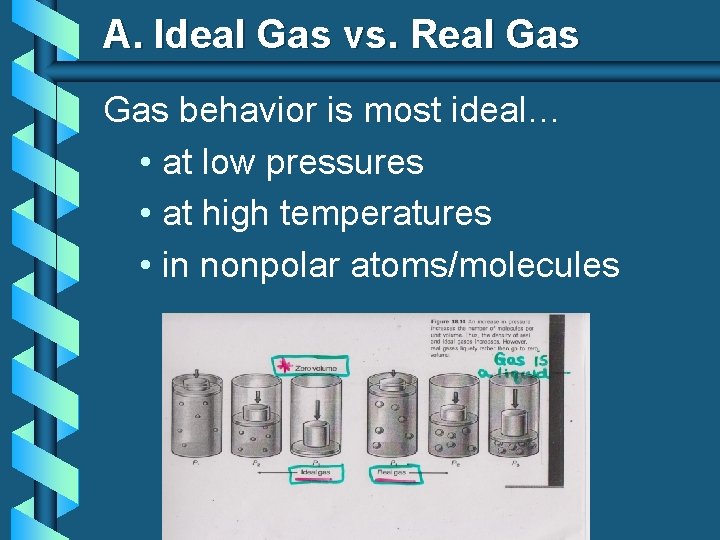
A. Ideal Gas vs. Real Gas behavior is most ideal… • at low pressures • at high temperatures • in nonpolar atoms/molecules Ø (see picture)

B. Characteristics of Gases 1. Gases expand to fill any container. 2. Gases are fluids (like liquids). 3. Gases can be compressed. 4. Gases exert pressure. 5. Gases undergo diffusion & effusion.

B. Characteristics of Gases b Diffusion • Spreading of gas molecules throughout a container until evenly distributed. b Effusion • Passing of gas molecules through a tiny opening in a container.

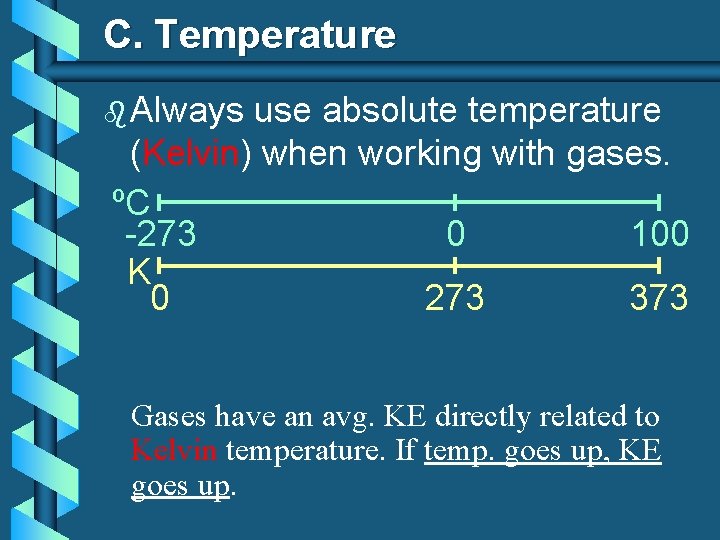
C. Temperature b Always use absolute temperature (Kelvin) when working with gases. ºC -273 0 100 K 0 273 373 Gases have an avg. KE directly related to Kelvin temperature. If temp. goes up, KE goes up.

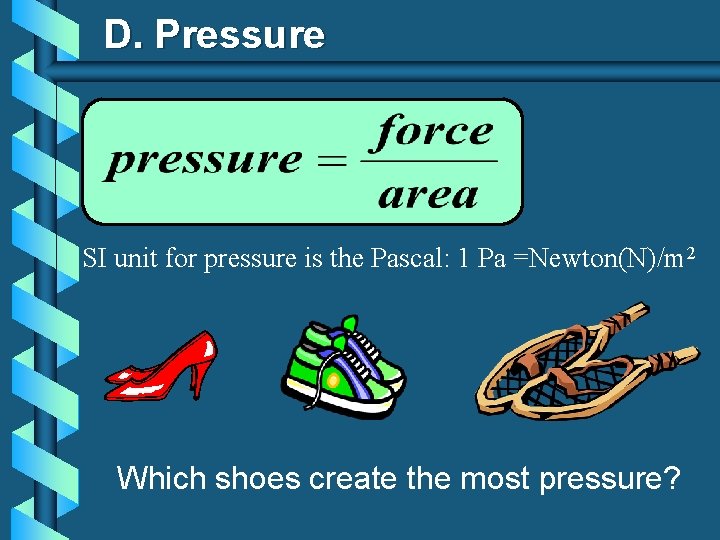
D. Pressure SI unit for pressure is the Pascal: 1 Pa =Newton(N)/m 2 Which shoes create the most pressure?

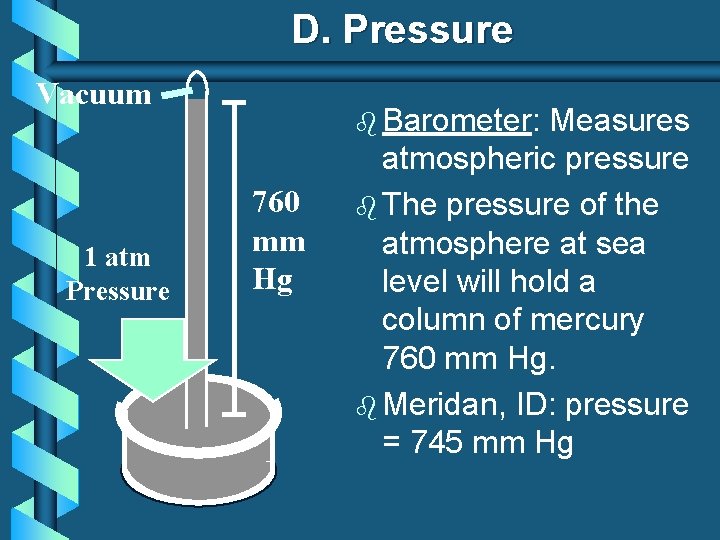
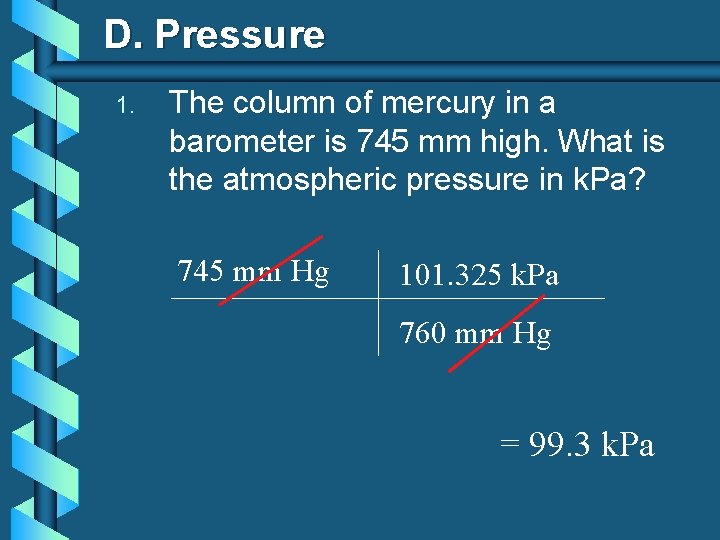
D. Pressure Vacuum 1 atm Pressure b Barometer: 760 mm Hg Measures atmospheric pressure b The pressure of the atmosphere at sea level will hold a column of mercury 760 mm Hg. b Meridan, ID: pressure = 745 mm Hg

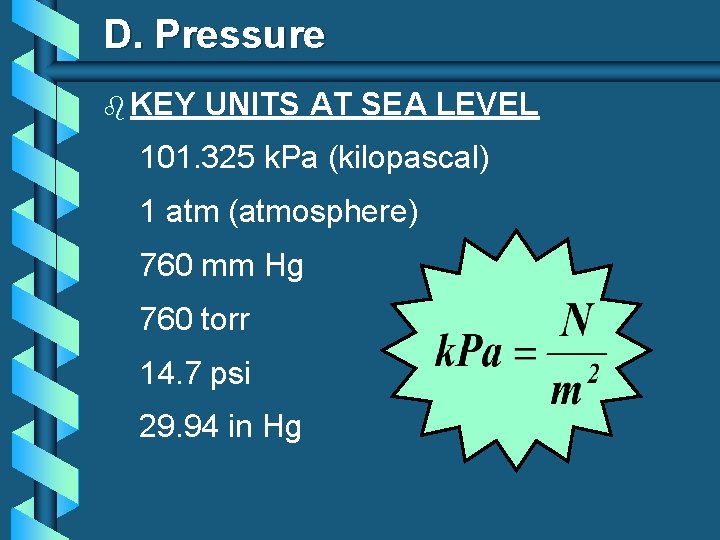
D. Pressure b KEY UNITS AT SEA LEVEL 101. 325 k. Pa (kilopascal) 1 atm (atmosphere) 760 mm Hg 760 torr 14. 7 psi 29. 94 in Hg

D. Pressure 1. The column of mercury in a barometer is 745 mm high. What is the atmospheric pressure in k. Pa? 745 mm Hg 101. 325 k. Pa 760 mm Hg = 99. 3 k. Pa

E. STP Standard Temperature & Pressure 0°C Or 273 K 1 atm Or 101. 325 k. Pa Or …

F. The Gas Laws: A series of mathematical relationships that describe the behavior of gases in regards to Pressure, Volume, & Temperature

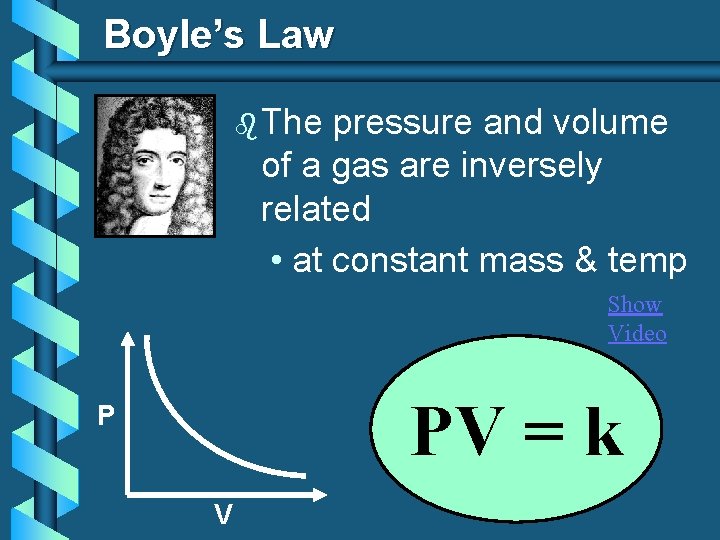
Boyle’s Law b The pressure and volume of a gas are inversely related • at constant mass & temp Show Video PV = k P V

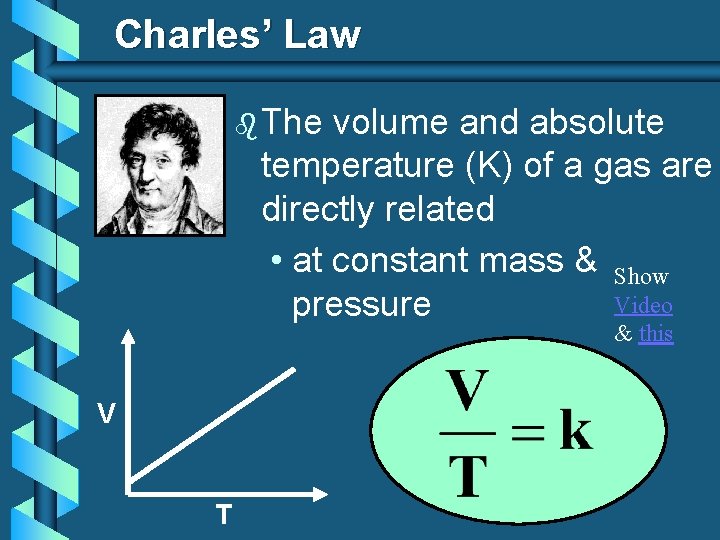
Charles’ Law b The volume and absolute temperature (K) of a gas are directly related • at constant mass & Show Video pressure & this V T

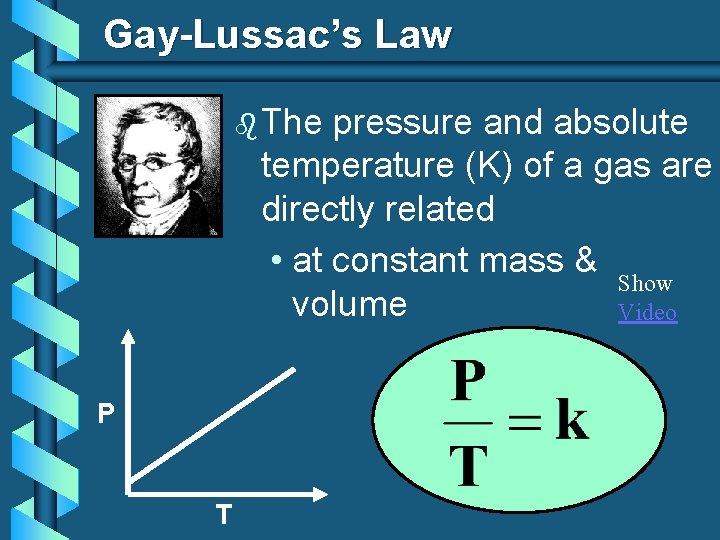
Gay-Lussac’s Law b The pressure and absolute temperature (K) of a gas are directly related • at constant mass & Show volume Video P T

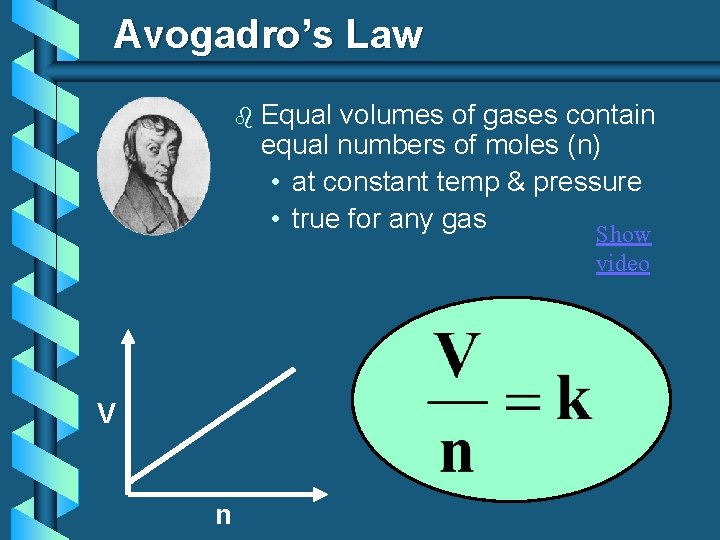
Avogadro’s Law b Equal volumes of gases contain equal numbers of moles (n) • at constant temp & pressure • true for any gas Show video V n

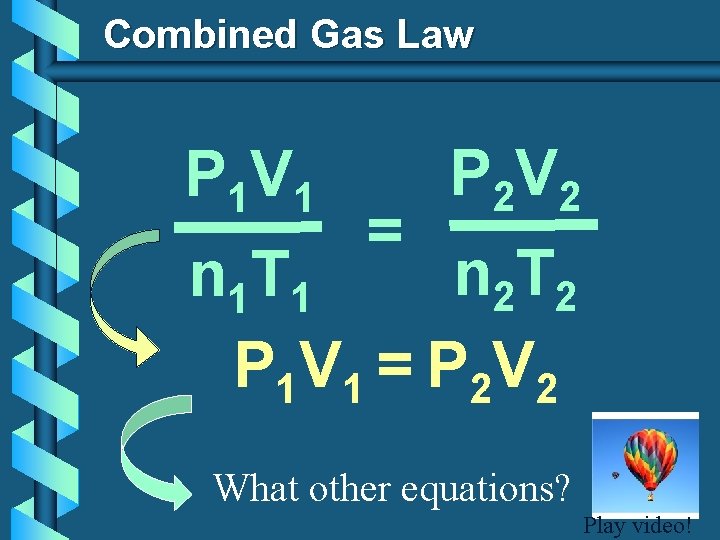
Combined Gas Law P 1 V 1 = P 2 V 2 n 2 T 2 n 1 T 1 P 1 V 1 = P 2 V 2 What other equations? Play video!

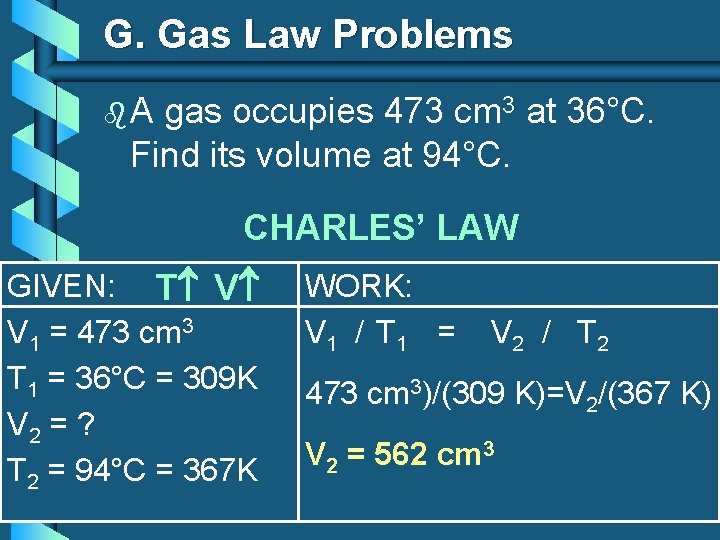
G. Gas Law Problems b. A gas occupies 473 cm 3 at 36°C. Find its volume at 94°C. CHARLES’ LAW GIVEN: T V V 1 = 473 cm 3 T 1 = 36°C = 309 K V 2 = ? T 2 = 94°C = 367 K WORK: V 1 / T 1 = V 2 / T 2 473 cm 3)/(309 K)=V 2/(367 K) V 2 = 562 cm 3

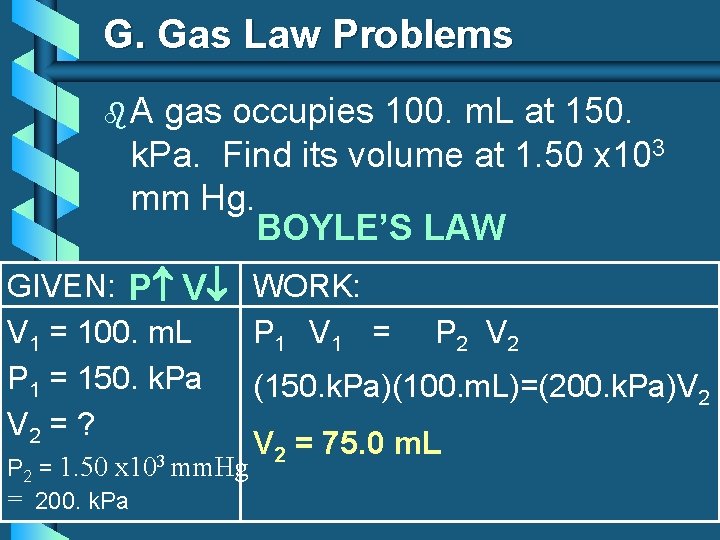
G. Gas Law Problems b. A gas occupies 100. m. L at 150. k. Pa. Find its volume at 1. 50 x 103 mm Hg. BOYLE’S LAW GIVEN: P V WORK: V 1 = 100. m. L P 1 V 1 = P 2 V 2 P 1 = 150. k. Pa (150. k. Pa)(100. m. L)=(200. k. Pa)V 2 = ? V 2 = 75. 0 m. L 3 P 2 = 1. 50 x 10 mm. Hg = 200. k. Pa

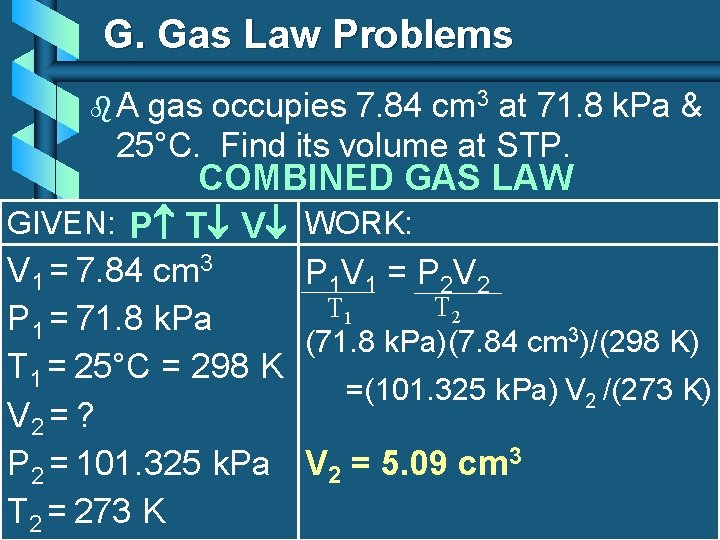
G. Gas Law Problems b. A gas occupies 7. 84 cm 3 at 71. 8 k. Pa & 25°C. Find its volume at STP. COMBINED GAS LAW GIVEN: P T V WORK: V 1 = 7. 84 cm 3 P 1 V 1 = P 2 V 2 T 1 P 1 = 71. 8 k. Pa (71. 8 k. Pa)(7. 84 cm 3)/(298 K) T 1 = 25°C = 298 K =(101. 325 k. Pa) V 2 /(273 K) V 2 = ? P 2 = 101. 325 k. Pa V 2 = 5. 09 cm 3 T 2 = 273 K
 What are the different properties of gas?
What are the different properties of gas? Chemical property of matter
Chemical property of matter Kinetic theory for ideal gases
Kinetic theory for ideal gases Characteristics of ideal gases
Characteristics of ideal gases Ideal gas characteristics
Ideal gas characteristics Are ideal gases compressible
Are ideal gases compressible First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas Buoyancyability
Buoyancyability What are the properties of solids
What are the properties of solids Properties of gas
Properties of gas Four properties of gases
Four properties of gases 5 properties of gases
5 properties of gases Properties of gases
Properties of gases General properties of noble gases
General properties of noble gases Gases have low densities
Gases have low densities Chemical properties of noble gases
Chemical properties of noble gases Liquid information
Liquid information State avogadro's law
State avogadro's law Properties of gas
Properties of gas List 2 of the important properties of gases
List 2 of the important properties of gases Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases