Characteristics of Gases Physical properties of gases are








- Slides: 56

Characteristics of Gases • Physical properties of gases are all similar. • Composed mainly of nonmetallic elements with simple formulas and low molar masses. • Unlike liquids and solids, gases Ø expand to fill their containers. Ø are highly compressible. Ø have extremely low densities. • Two or more gases form a homogeneous mixture. Gases © 2015 Pearson Education, Inc. 1 גזים 8 -

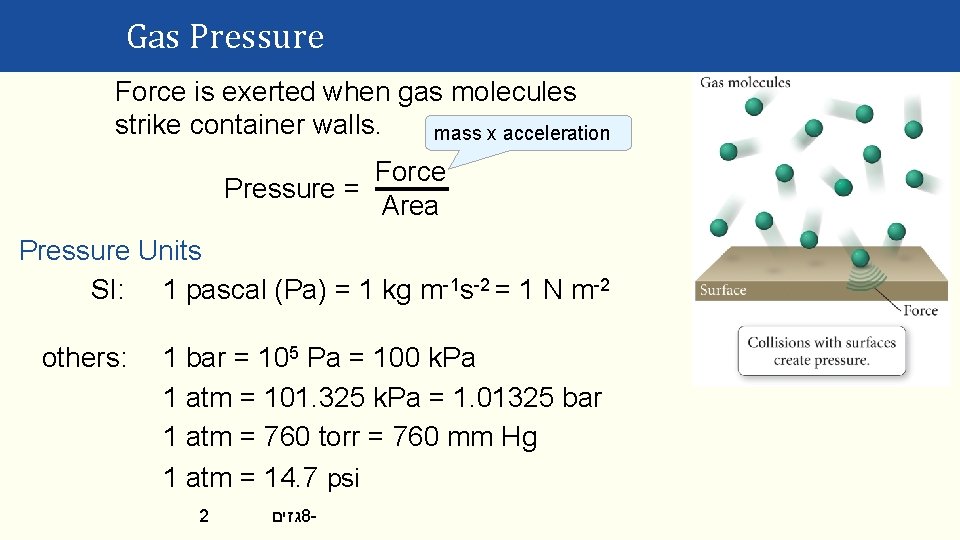
Gas Pressure Force is exerted when gas molecules strike container walls. mass x acceleration Force Pressure = Area Pressure Units SI: 1 pascal (Pa) = 1 kg m-1 s-2 = 1 N m-2 others: 1 bar = 105 Pa = 100 k. Pa 1 atm = 101. 325 k. Pa = 1. 01325 bar 1 atm = 760 torr = 760 mm Hg 1 atm = 14. 7 psi 2 גזים 8 -

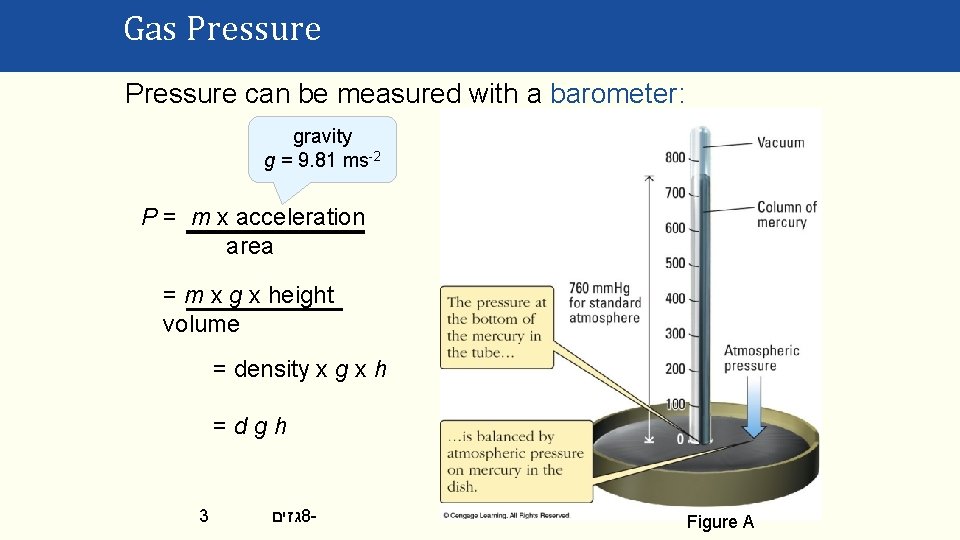
Gas Pressure can be measured with a barometer: gravity g = 9. 81 ms-2 P = m x acceleration area = m x g x height volume = density x g x h =dgh 3 גזים 8 - Figure A

Pressure Suppose that you set up two barometers like the one shown in Figure A. In one of the barometers you use mercury, and in the other you use water. Which of the barometers would have a higher column of liquid, the one with Hg or H 2 O? Explain your answer. The water column would be higher because its density is less by a factor equal to the density of mercury to the density of water. © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5|4

Standard Pressure • Normal atmospheric pressure at sea level is referred to as standard atmospheric pressure. • It is equal to Ø 1. 00 atm. Ø 760 torr (760 mm. Hg). Ø 101. 325 k. Pa. – Pressure decreases with increasing altitude. 5 גזים 8 -

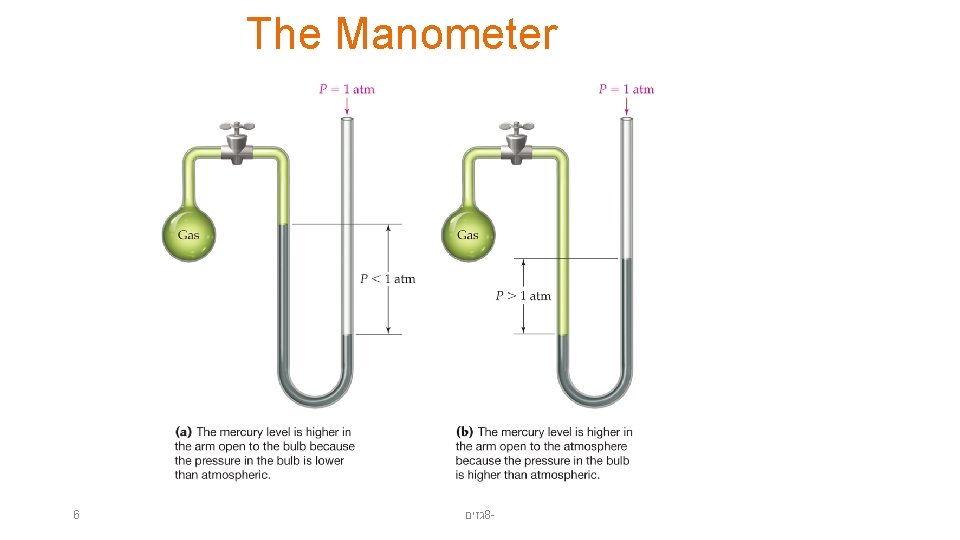
The Manometer 6 גזים 8 -

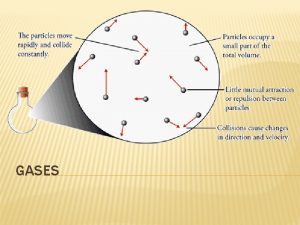
Kinetic-Molecular Theory Gas molecules: 1. are small compared to the distances between them • easily compressed. • mix completely with other gases. 2. move randomly at very high speeds. • quickly and completely fill any container. 3. have small attractions/repulsions for each other. • all gases behave the same way. 4. make elastic collisions with each other. • don’t slow over time & fall to bottom of container. 5. have kinetic energy proportional to absolute T. 7 גזים 8 -

The Behavior of Ideal Gases: Gas Laws Any equation relating volume (V), pressure (P), temperature (T ) and/or the amount of gas, in moles, (n) is called a gas law. Most gases at room T and P are ideal; they follow a simple set of gas laws. 8 גזים 8 -

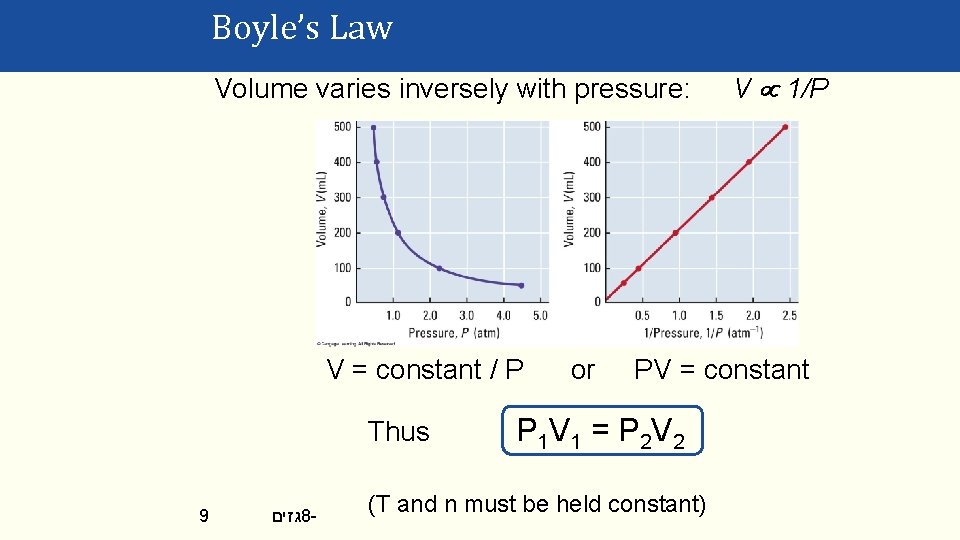
Boyle’s Law Volume varies inversely with pressure: V = constant / P Thus 9 גזים 8 - or V 1/P PV = constant P 1 V 1 = P 2 V 2 (T and n must be held constant)

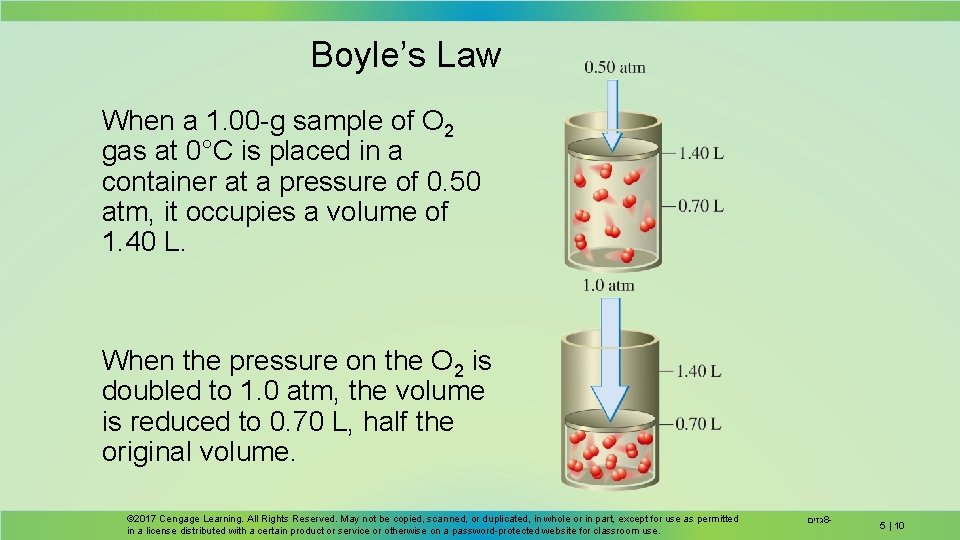
Boyle’s Law When a 1. 00 -g sample of O 2 gas at 0°C is placed in a container at a pressure of 0. 50 atm, it occupies a volume of 1. 40 L. When the pressure on the O 2 is doubled to 1. 0 atm, the volume is reduced to 0. 70 L, half the original volume. © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 10

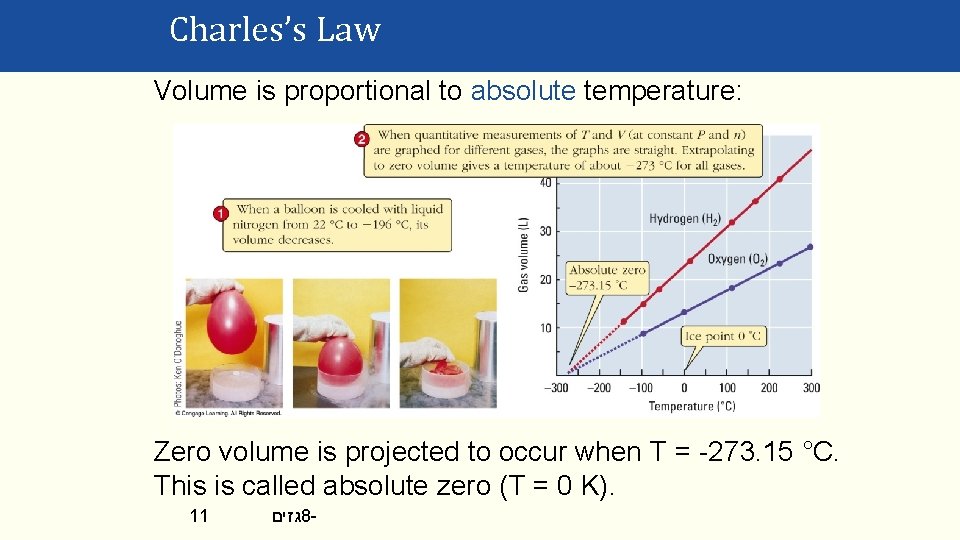
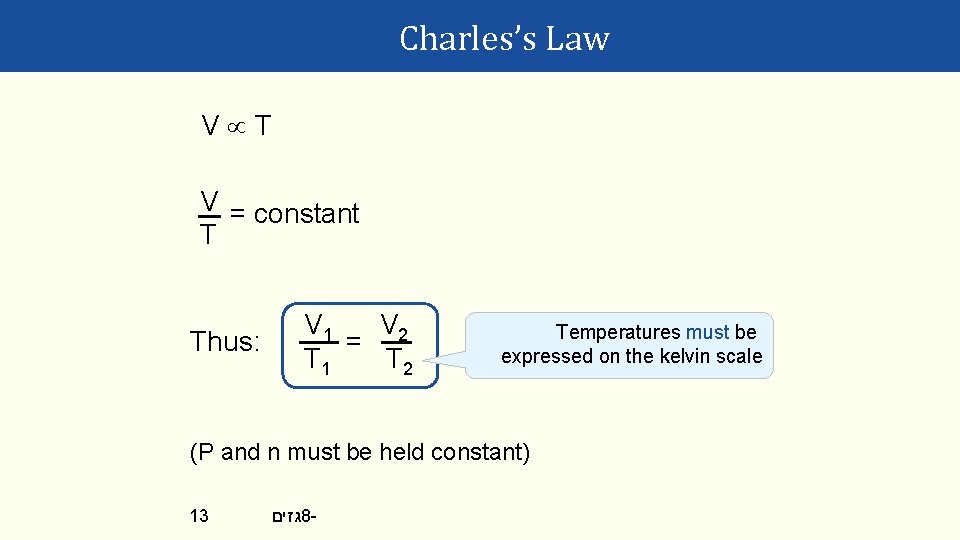
Charles’s Law Volume is proportional to absolute temperature: Zero volume is projected to occur when T = -273. 15 °C. This is called absolute zero (T = 0 K). 11 גזים 8 -

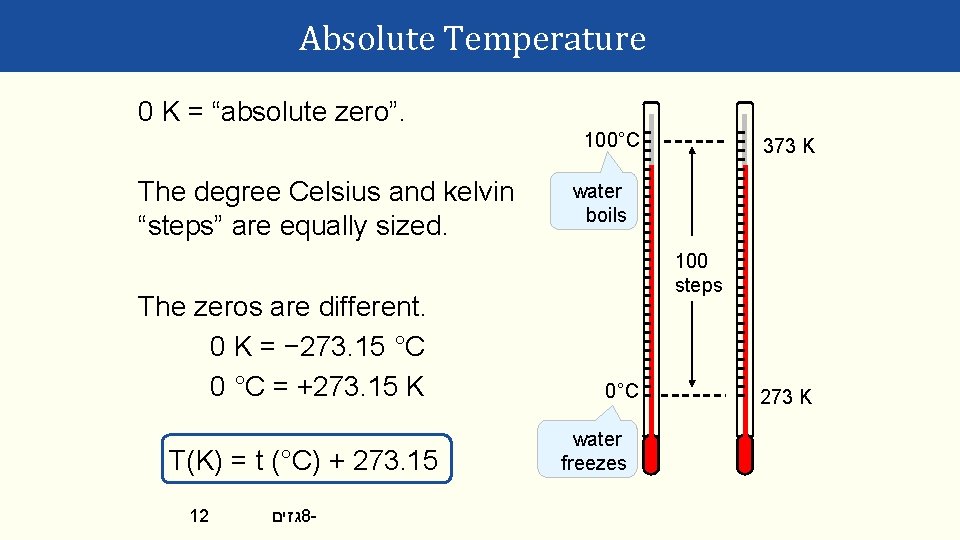
Absolute Temperature 0 K = “absolute zero”. 100°C The degree Celsius and kelvin “steps” are equally sized. The zeros are different. 0 K = − 273. 15 °C 0 °C = +273. 15 K T(K) = t (°C) + 273. 15 12 גזים 8 - 373 K water boils 100 steps 0°C water freezes 273 K

Charles’s Law V T V = constant T Thus: V 1 V 2 = T 1 T 2 Temperatures must be expressed on the kelvin scale (P and n must be held constant) 13 גזים 8 -

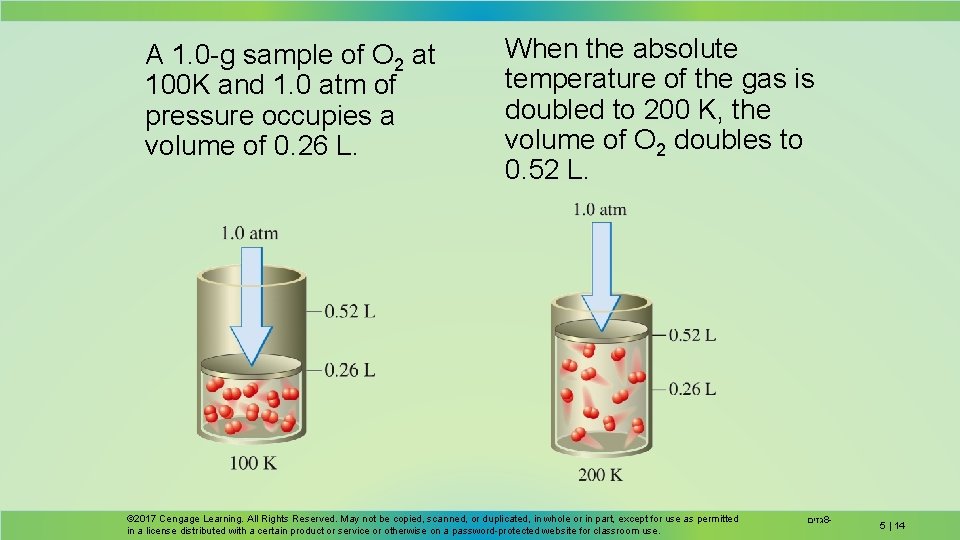
A 1. 0 -g sample of O 2 at 100 K and 1. 0 atm of pressure occupies a volume of 0. 26 L. When the absolute temperature of the gas is doubled to 200 K, the volume of O 2 doubles to 0. 52 L. © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 14

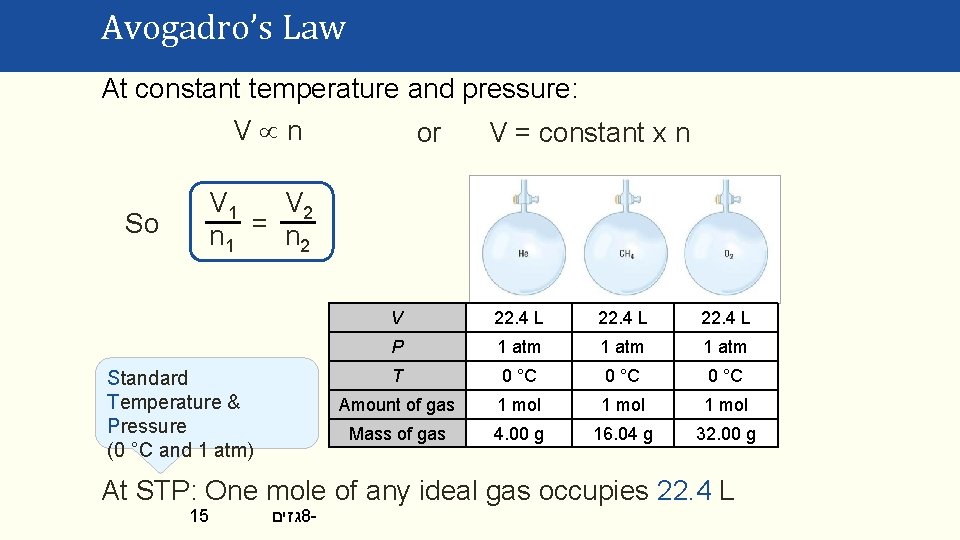
Avogadro’s Law At constant temperature and pressure: V n or V = constant x n V 1 V 2 n 1 = n 2 So Standard Temperature & Pressure (0 °C and 1 atm) V 22. 4 L P 1 atm T 0 °C Amount of gas 1 mol Mass of gas 4. 00 g 16. 04 g 32. 00 g At STP: One mole of any ideal gas occupies 22. 4 L 15 גזים 8 -

Standard Temperature and Pressure (STP) The reference conditions for gases chosen by convention to be exactly 0°C and 1 atm pressure. The molar volume of a gas at STP is 22. 4 L/mol. The volume of the yellow box is 22. 4 L. To its left is a basketball. © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 16

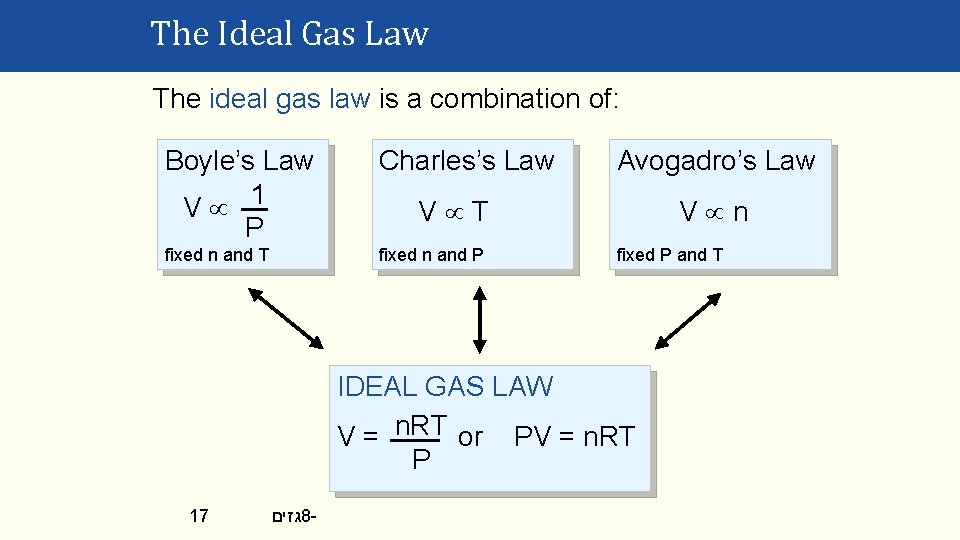
The Ideal Gas Law The ideal gas law is a combination of: Boyle’s Law 1 V P Charles’s Law fixed n and T fixed n and P Avogadro’s Law V n V T fixed P and T IDEAL GAS LAW V = n. RT or PV = n. RT P 17 גזים 8 -

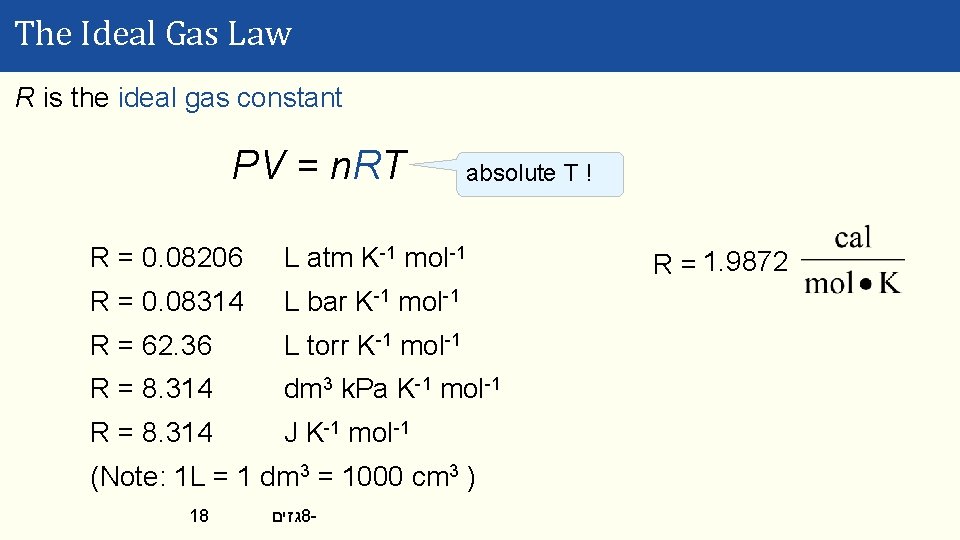
The Ideal Gas Law R is the ideal gas constant PV = n. RT absolute T ! R = 0. 08206 L atm K-1 mol-1 R = 0. 08314 L bar K-1 mol-1 R = 62. 36 L torr K-1 mol-1 R = 8. 314 dm 3 k. Pa K-1 mol-1 R = 8. 314 J K-1 mol-1 (Note: 1 L = 1 dm 3 = 1000 cm 3 ) 18 גזים 8 - R = 1. 9872

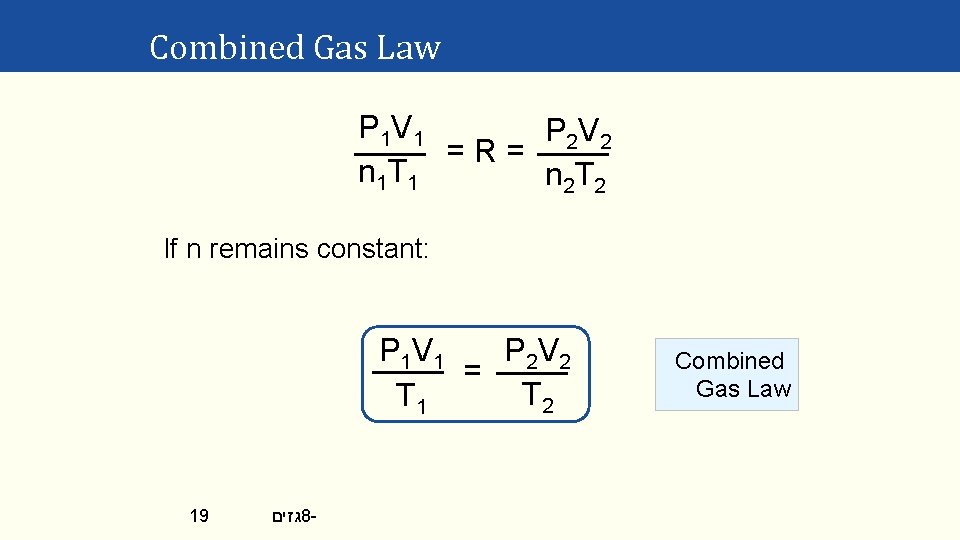
Combined Gas Law P 1 V 1 P 2 V 2 =R= n 1 T 1 n 2 T 2 If n remains constant: P 2 V 2 P 1 V 1 = T 2 T 1 19 גזים 8 - Combined Gas Law

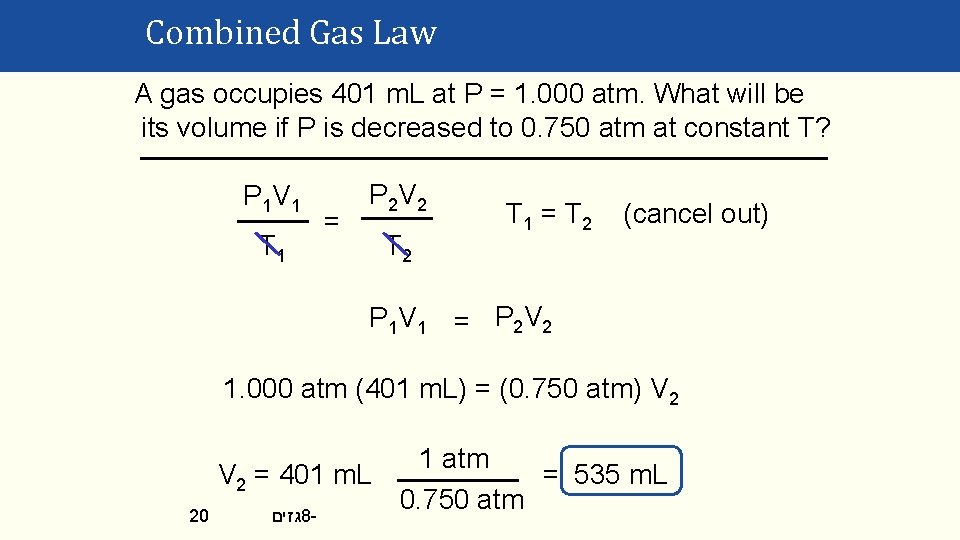
Combined Gas Law A gas occupies 401 m. L at P = 1. 000 atm. What will be its volume if P is decreased to 0. 750 atm at constant T? P 1 V 1 T 1 = P 2 V 2 T 1 = T 2 (cancel out) P 1 V 1 = P 2 V 2 1. 000 atm (401 m. L) = (0. 750 atm) V 2 20 1 atm V 2 = 401 m. L = 535 m. L 0. 750 atm גזים 8 -

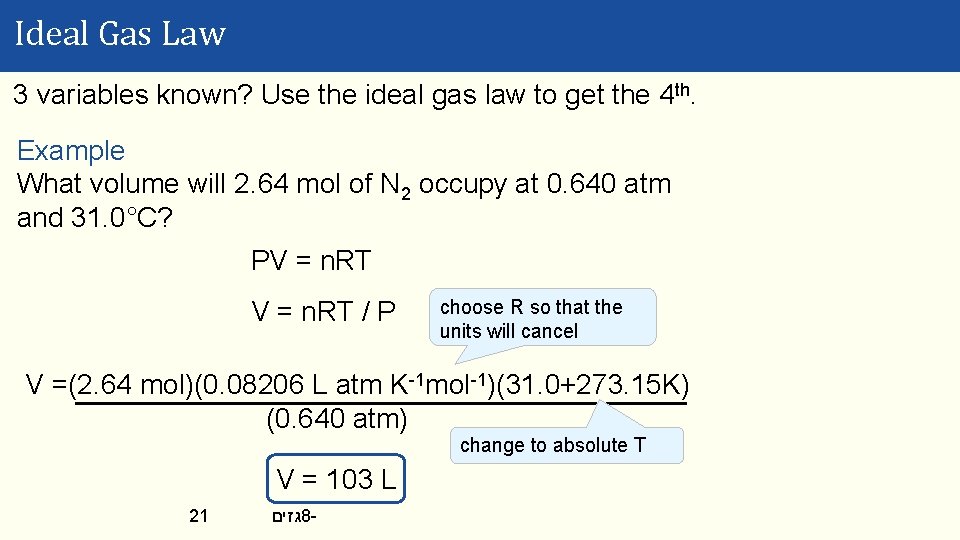
Ideal Gas Law 3 variables known? Use the ideal gas law to get the 4 th. Example What volume will 2. 64 mol of N 2 occupy at 0. 640 atm and 31. 0°C? PV = n. RT / P choose R so that the units will cancel V =(2. 64 mol)(0. 08206 L atm K-1 mol-1)(31. 0+273. 15 K) (0. 640 atm) change to absolute T V = 103 L 21 גזים 8 -

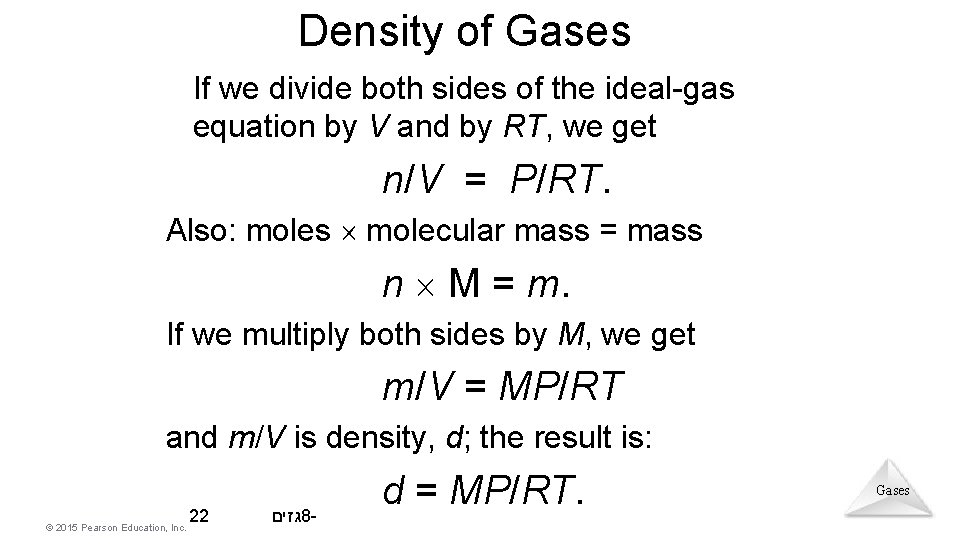
Density of Gases If we divide both sides of the ideal-gas equation by V and by RT, we get n/V = P/RT. Also: moles molecular mass = mass n M = m. If we multiply both sides by M, we get m/V = MP/RT and m/V is density, d; the result is: © 2015 Pearson Education, Inc. 22 גזים 8 - d = MP/RT. Gases

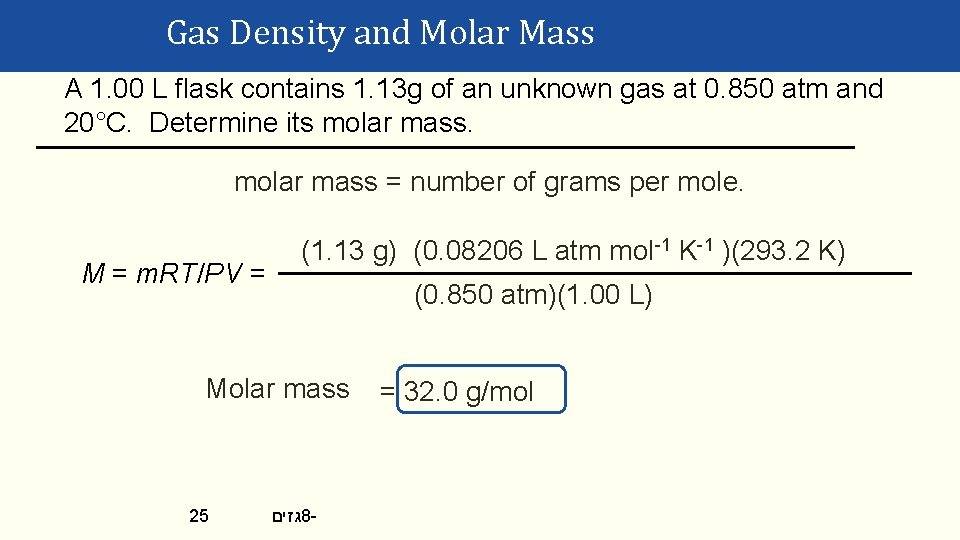
Density & Molar Mass of a Gas • To recap: • One needs to know only the molecular mass, the pressure, and the temperature to calculate the density of a gas. • d = MP/RT • Also, if we know the mass, volume, and temperature of a gas, we can find its molar mass. • M = m. RT/PV Gases © 2015 Pearson Education, Inc. 23 גזים 8 -

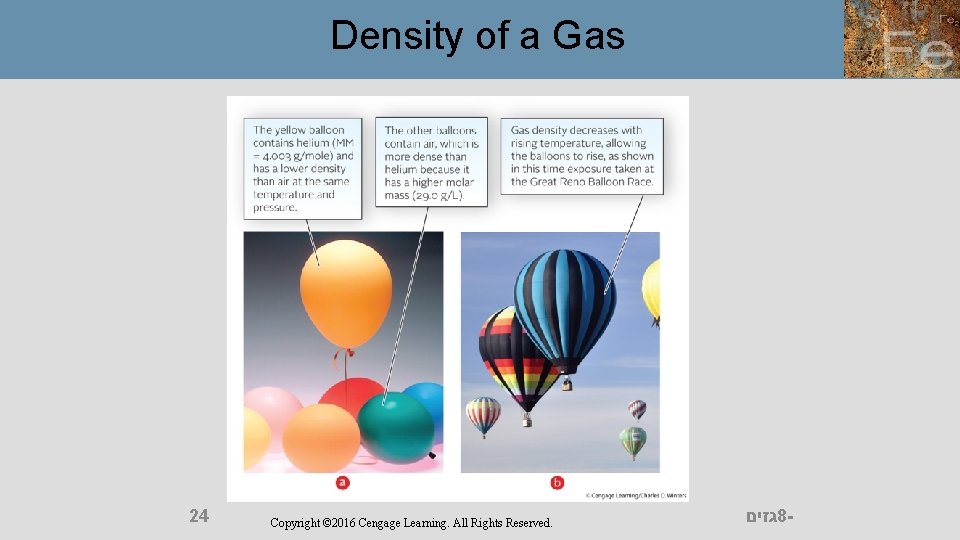
Density of a Gas 24 Copyright © 2016 Cengage Learning. All Rights Reserved. גזים 8 -

Gas Density and Molar Mass A 1. 00 L flask contains 1. 13 g of an unknown gas at 0. 850 atm and 20°C. Determine its molar mass = number of grams per mole. M = m. RT/PV = (1. 13 g) (0. 08206 L atm mol-1 K-1 )(293. 2 K) (0. 850 atm)(1. 00 L) Molar mass 25 גזים 8 - = 32. 0 g/mol

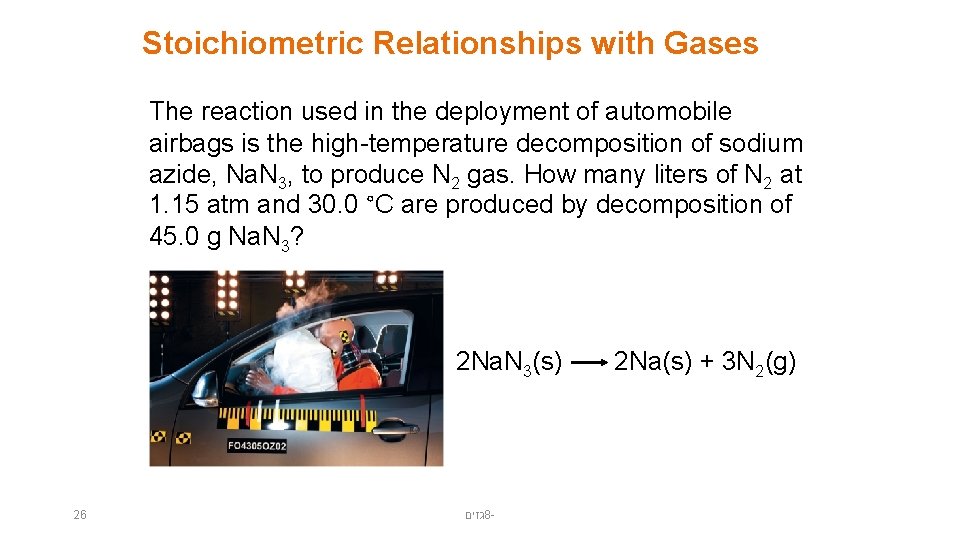
Stoichiometric Relationships with Gases The reaction used in the deployment of automobile airbags is the high-temperature decomposition of sodium azide, Na. N 3, to produce N 2 gas. How many liters of N 2 at 1. 15 atm and 30. 0 °C are produced by decomposition of 45. 0 g Na. N 3? 2 Na. N 3(s) 26 גזים 8 - 2 Na(s) + 3 N 2(g)

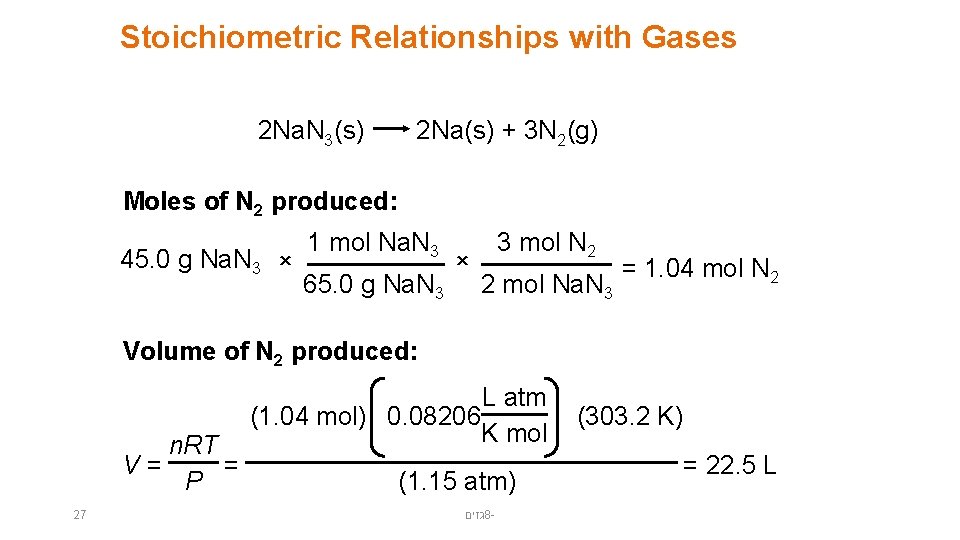
Stoichiometric Relationships with Gases 2 Na. N 3(s) 2 Na(s) + 3 N 2(g) Moles of N 2 produced: 45. 0 g Na. N 3 × 1 mol Na. N 3 65. 0 g Na. N 3 × 3 mol N 2 2 mol Na. N 3 = 1. 04 mol N 2 Volume of N 2 produced: n. RT V= = P 27 L atm (1. 04 mol) 0. 08206 K mol (1. 15 atm) גזים 8 - (303. 2 K) = 22. 5 L

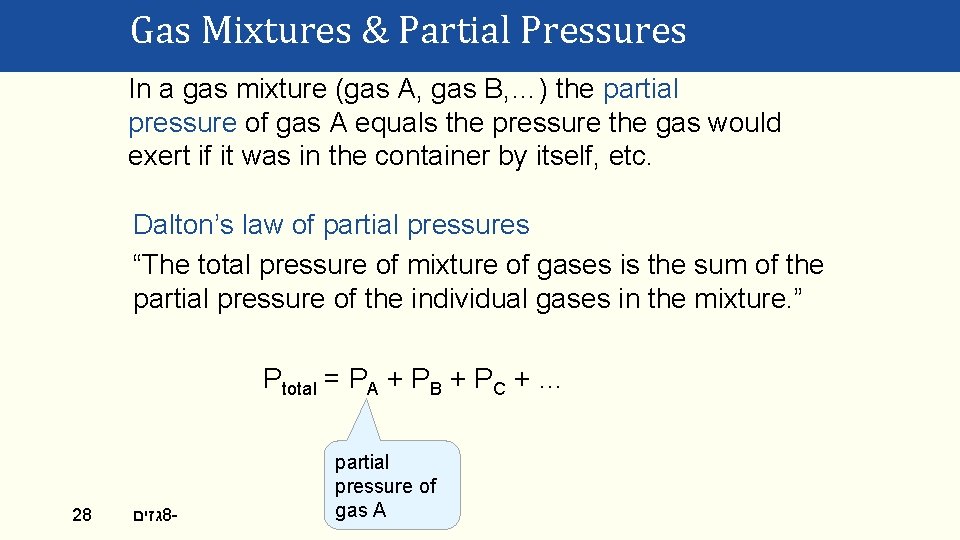
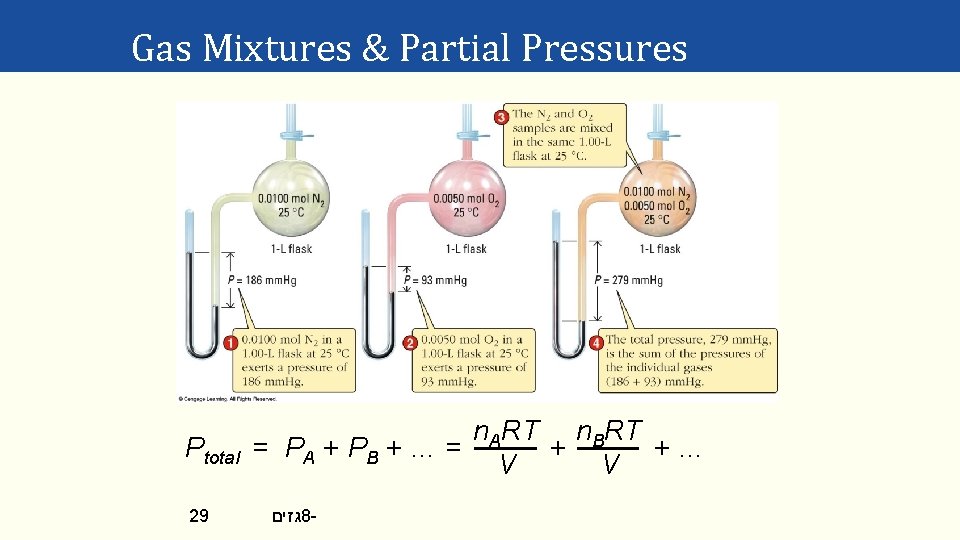
Gas Mixtures & Partial Pressures In a gas mixture (gas A, gas B, …) the partial pressure of gas A equals the pressure the gas would exert if it was in the container by itself, etc. Dalton’s law of partial pressures “The total pressure of mixture of gases is the sum of the partial pressure of the individual gases in the mixture. ” Ptotal = PA + PB + PC + … 28 גזים 8 - partial pressure of gas A

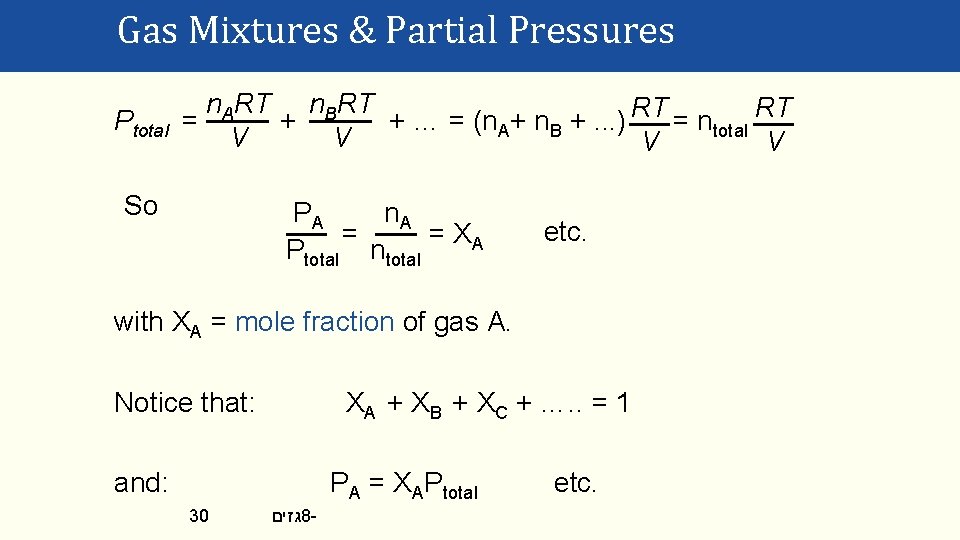
Gas Mixtures & Partial Pressures Ptotal 29 n. ART n. BRT = PA + PB + … = + +… V V גזים 8 -

Gas Mixtures & Partial Pressures Ptotal n. ART n. BRT = + + … = (n. A+ n. B +. . . ) RT = ntotal RT V V So PA n. A = = XA Ptotal ntotal etc. with XA = mole fraction of gas A. Notice that: XA + XB + XC + …. . = 1 and: PA = XAPtotal 30 גזים 8 - etc.

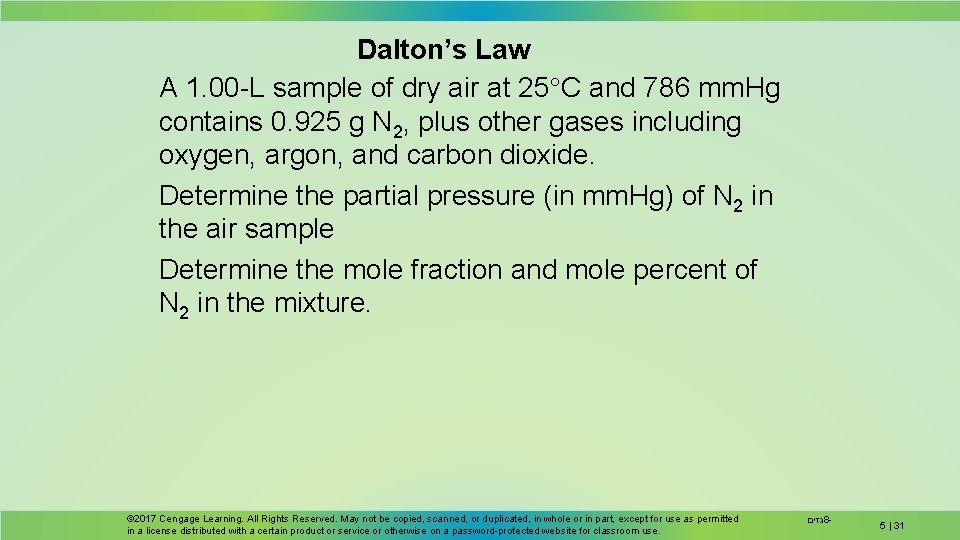
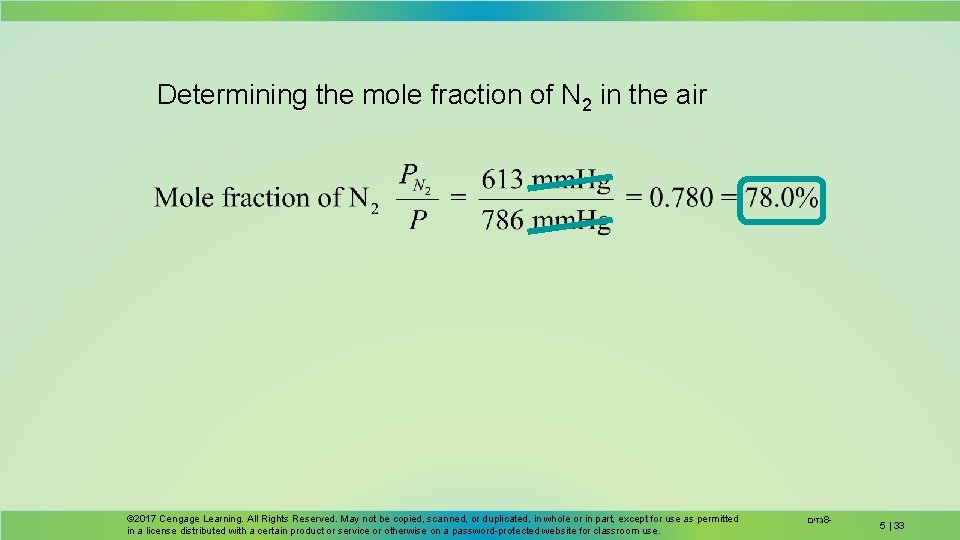
Dalton’s Law A 1. 00 -L sample of dry air at 25 C and 786 mm. Hg contains 0. 925 g N 2, plus other gases including oxygen, argon, and carbon dioxide. Determine the partial pressure (in mm. Hg) of N 2 in the air sample Determine the mole fraction and mole percent of N 2 in the mixture. © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 31

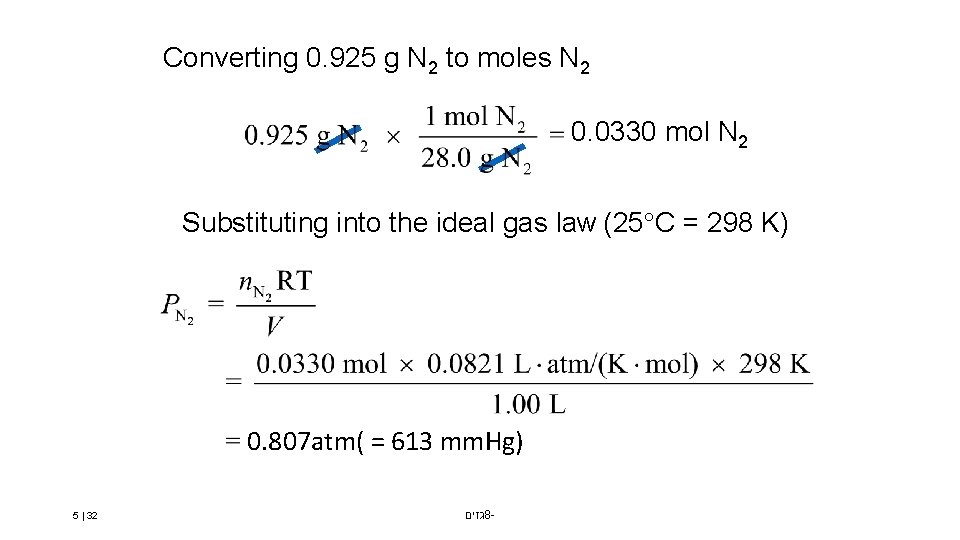
Converting 0. 925 g N 2 to moles N 2 0. 0330 mol N 2 Substituting into the ideal gas law (25 C = 298 K) 0. 807 atm( = 613 mm. Hg) 5 | 32 גזים 8 -

Determining the mole fraction of N 2 in the air © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 33

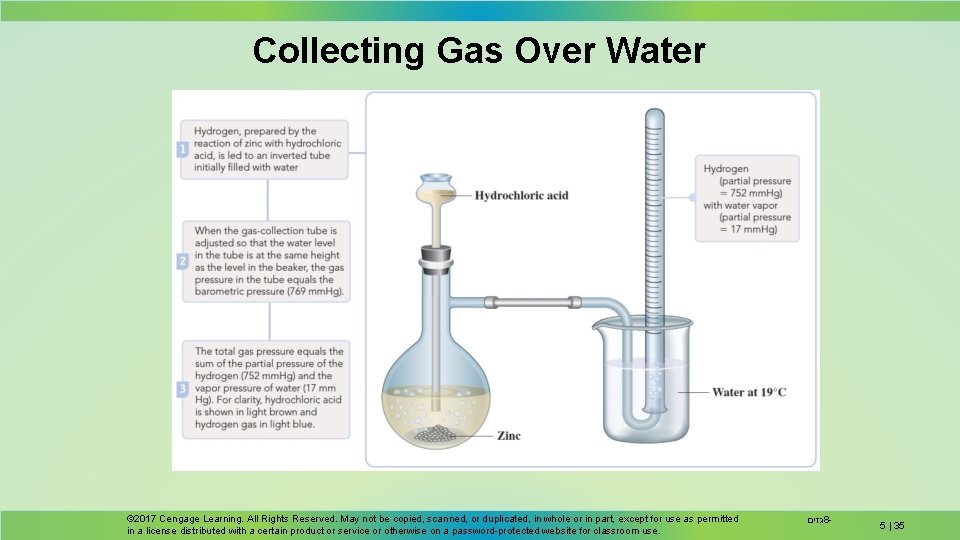
Collecting Gas Over Water Gases are often collected over water. The result is a mixture of the gas and water vapor. The partial pressure of water depends only on temperature. © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 34

Collecting Gas Over Water © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 35

Collecting a Gas by Water Displacement 36 Copyright © 2016 Cengage Learning. All Rights Reserved. גזים 8 -

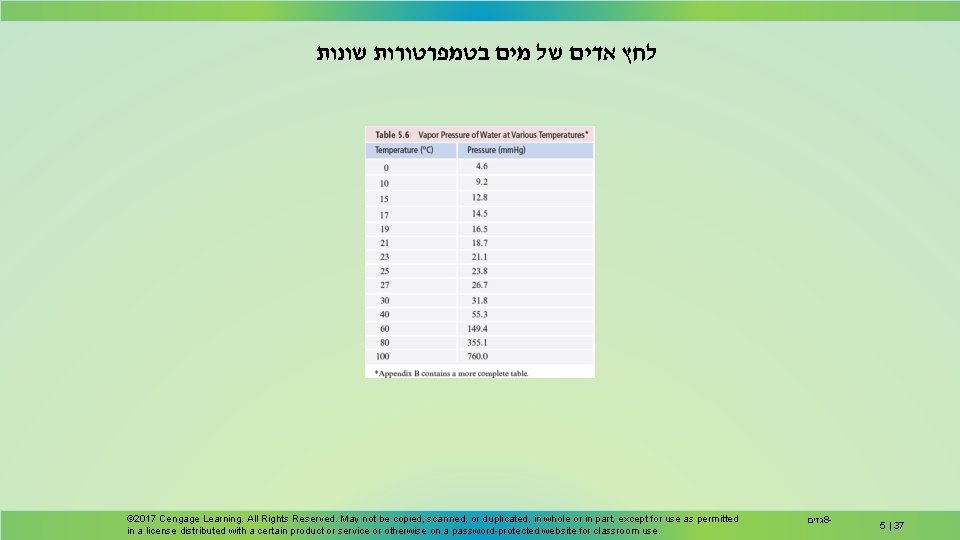
לחץ אדים של מים בטמפרטורות שונות © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 37

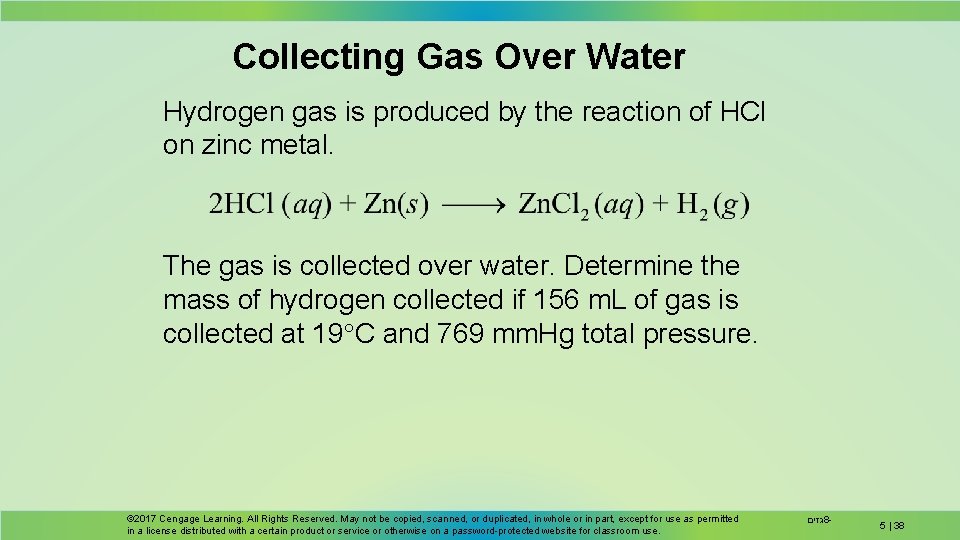
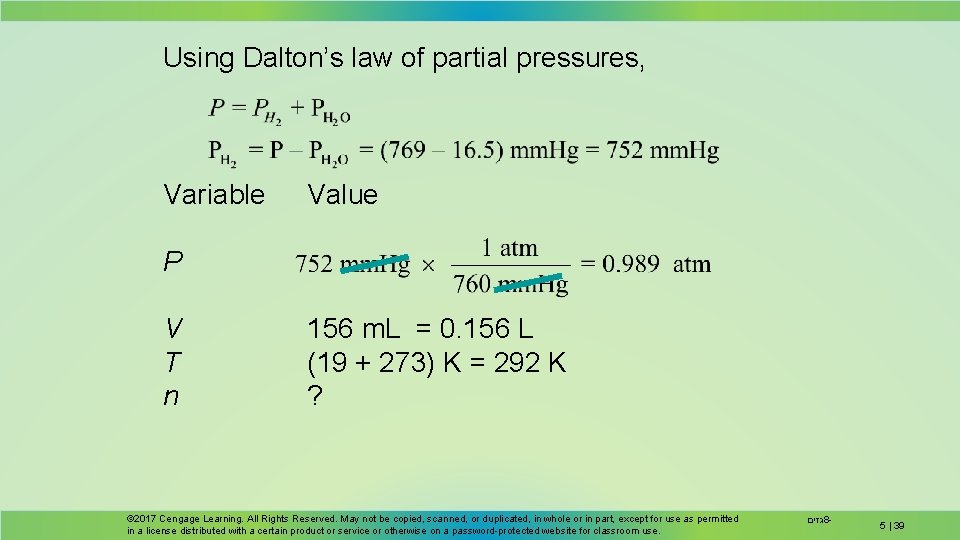
Collecting Gas Over Water Hydrogen gas is produced by the reaction of HCl on zinc metal. The gas is collected over water. Determine the mass of hydrogen collected if 156 m. L of gas is collected at 19 C and 769 mm. Hg total pressure. © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 38

Using Dalton’s law of partial pressures, Variable Value P V T n 156 m. L = 0. 156 L (19 + 273) K = 292 K ? © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 39

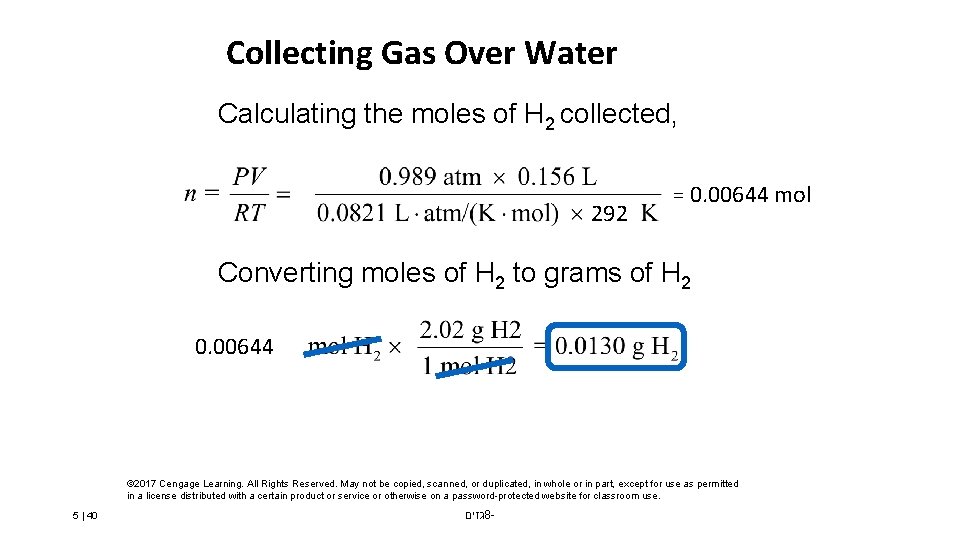
Collecting Gas Over Water Calculating the moles of H 2 collected, 292 = 0. 00644 mol Converting moles of H 2 to grams of H 2 0. 00644 © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. 5 | 40 גזים 8 -

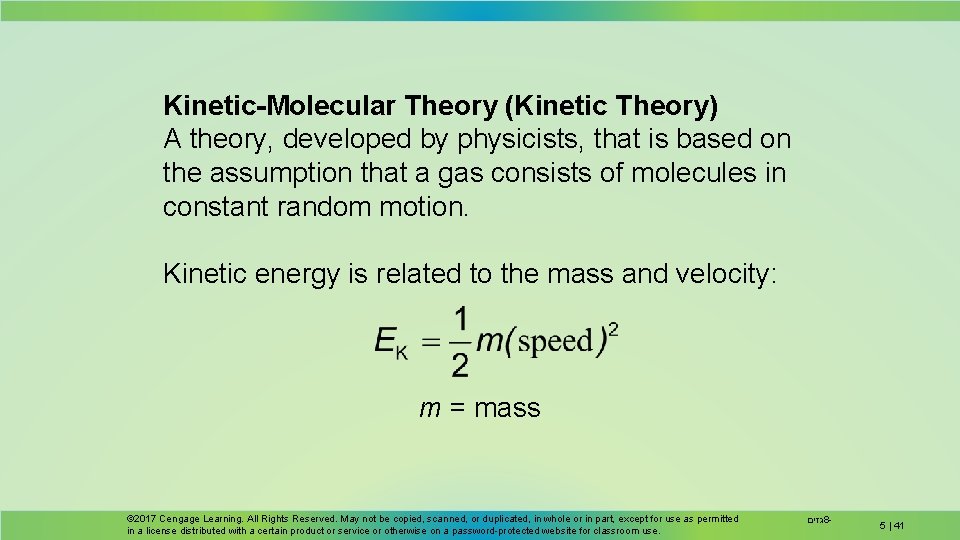
Kinetic-Molecular Theory (Kinetic Theory) A theory, developed by physicists, that is based on the assumption that a gas consists of molecules in constant random motion. Kinetic energy is related to the mass and velocity: m = mass © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 41

Postulates of the Kinetic Theory 1. Gases are composed of molecules whose size is negligible compared to the average distance between them 2. Molecules move randomly in straight lines in all directions and at various speeds. 3. The forces of attraction or repulsion between two molecules (intermolecular forces) in a gas are very weak or negligible, except when the molecules collide. 4. When molecules collide with each other, the collisions are elastic. 5. The average kinetic energy of a molecule is proportional to the absolute temperature. © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 42

An elastic collision is one in which no kinetic energy is lost. The collision on the left causes the ball on the right to swing the same height as the ball on the left had initially, with essentially no loss of kinetic energy. © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 43

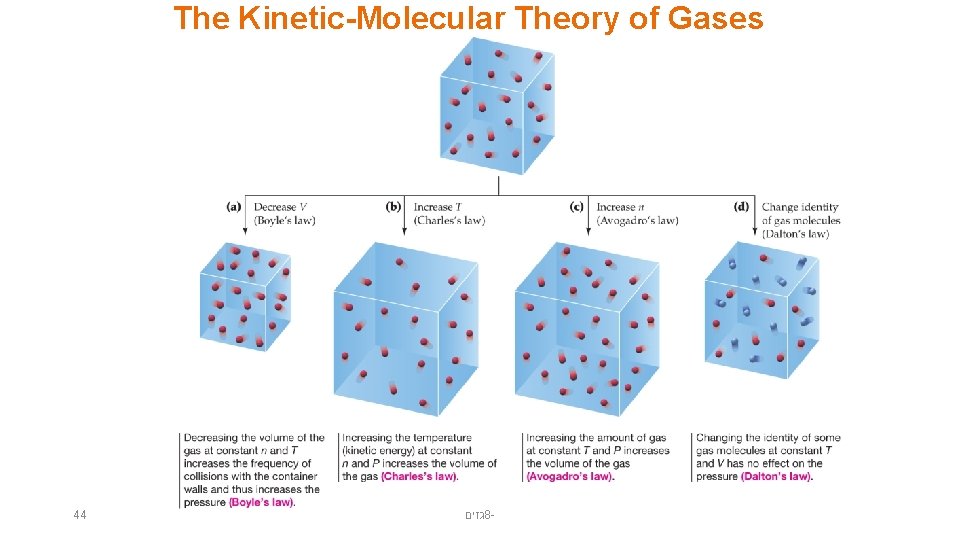
The Kinetic-Molecular Theory of Gases 44 גזים 8 -

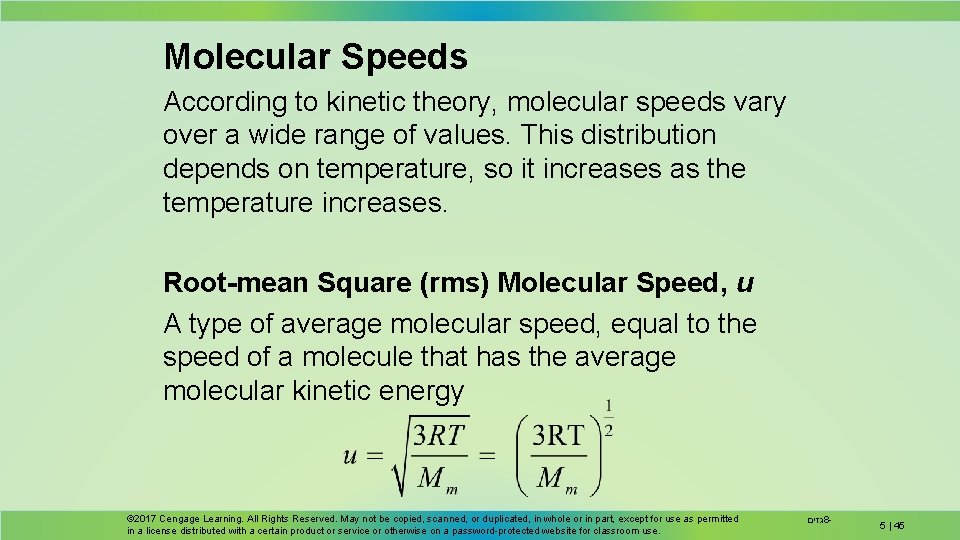
Molecular Speeds According to kinetic theory, molecular speeds vary over a wide range of values. This distribution depends on temperature, so it increases as the temperature increases. Root-mean Square (rms) Molecular Speed, u A type of average molecular speed, equal to the speed of a molecule that has the average molecular kinetic energy © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 45

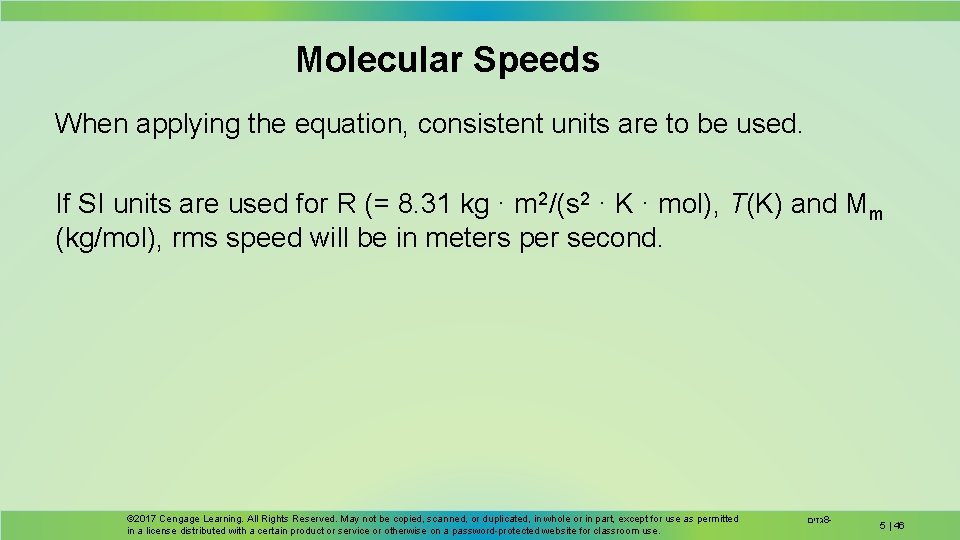
Molecular Speeds When applying the equation, consistent units are to be used. If SI units are used for R (= 8. 31 kg · m 2/(s 2 · K · mol), T(K) and Mm (kg/mol), rms speed will be in meters per second. © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 46

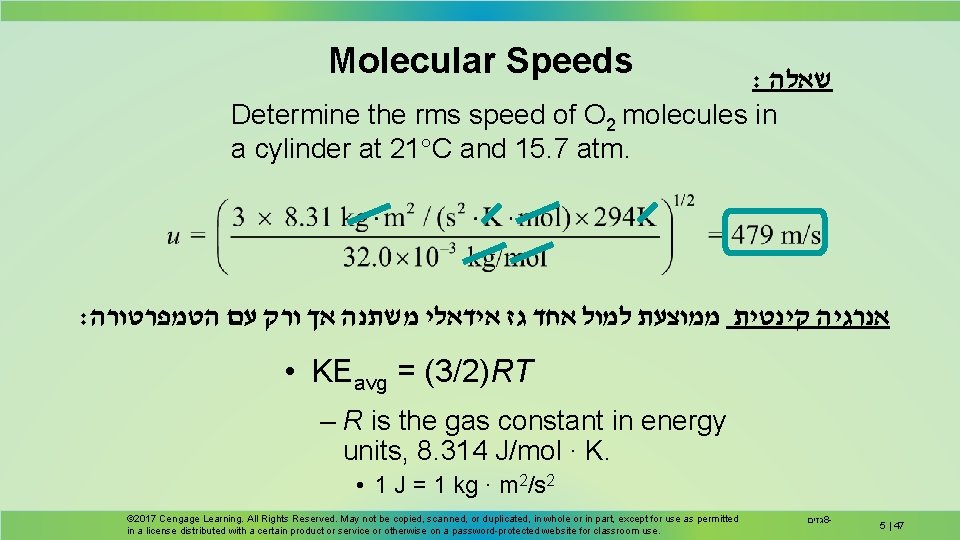
Molecular Speeds : שאלה Determine the rms speed of O 2 molecules in a cylinder at 21 C and 15. 7 atm. : אנרגיה קינטית ממוצעת למול אחד גז אידאלי משתנה אך ורק עם הטמפרטורה • KEavg = (3/2)RT – R is the gas constant in energy units, 8. 314 J/mol ∙ K. • 1 J = 1 kg ∙ m 2/s 2 © 2017 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part, except for use as permitted in a license distributed with a certain product or service or otherwise on a password-protected website for classroom use. גזים 8 - 5 | 47

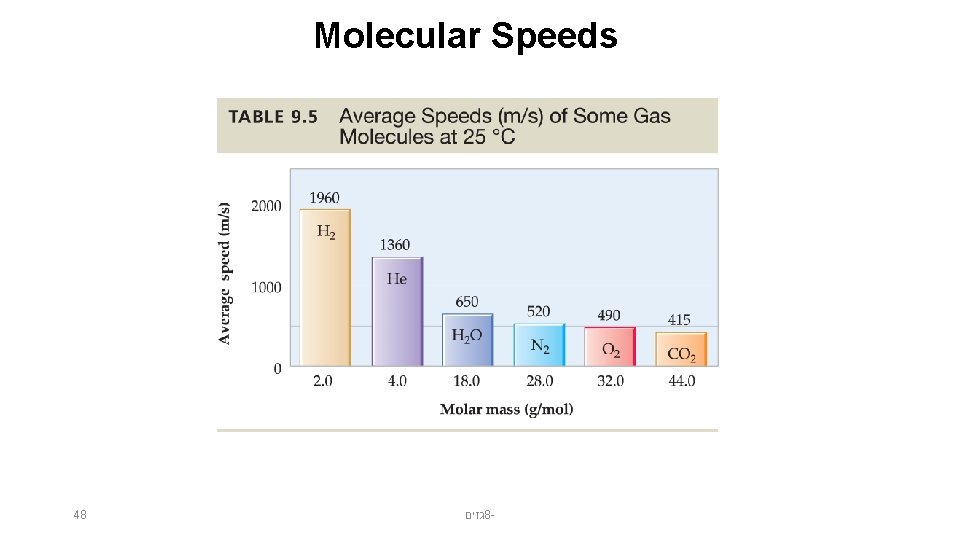
Molecular Speeds 48 גזים 8 -

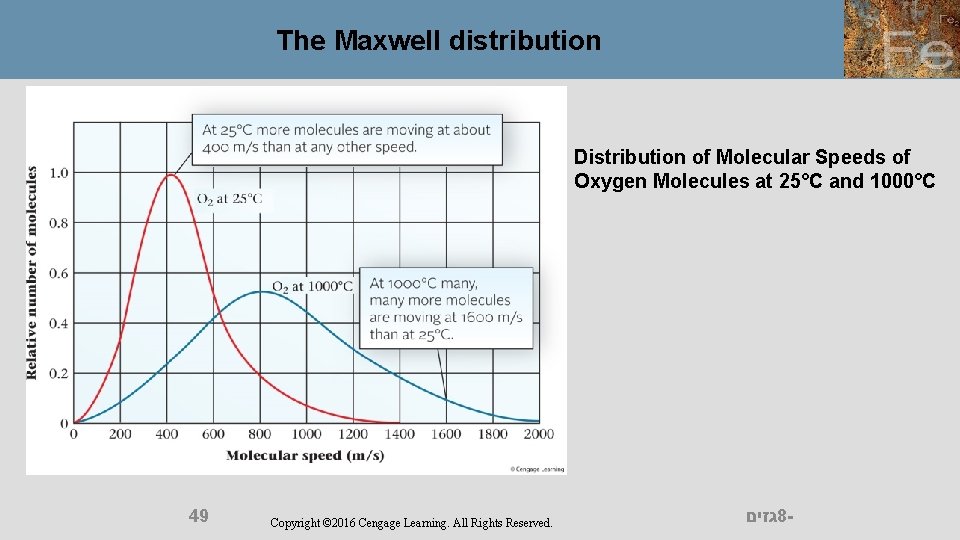
The Maxwell distribution Distribution of Molecular Speeds of Oxygen Molecules at 25°C and 1000°C 49 Copyright © 2016 Cengage Learning. All Rights Reserved. גזים 8 -

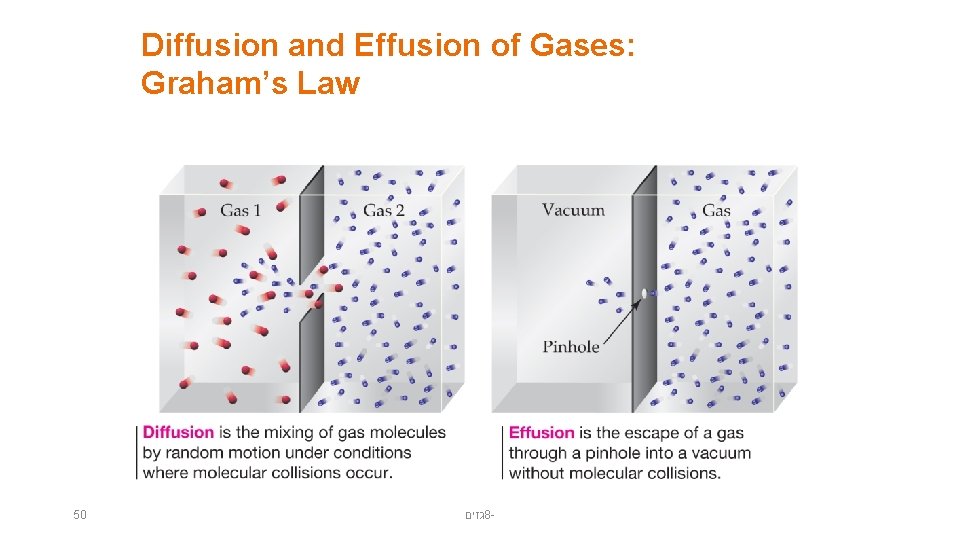
Diffusion and Effusion of Gases: Graham’s Law 50 גזים 8 -

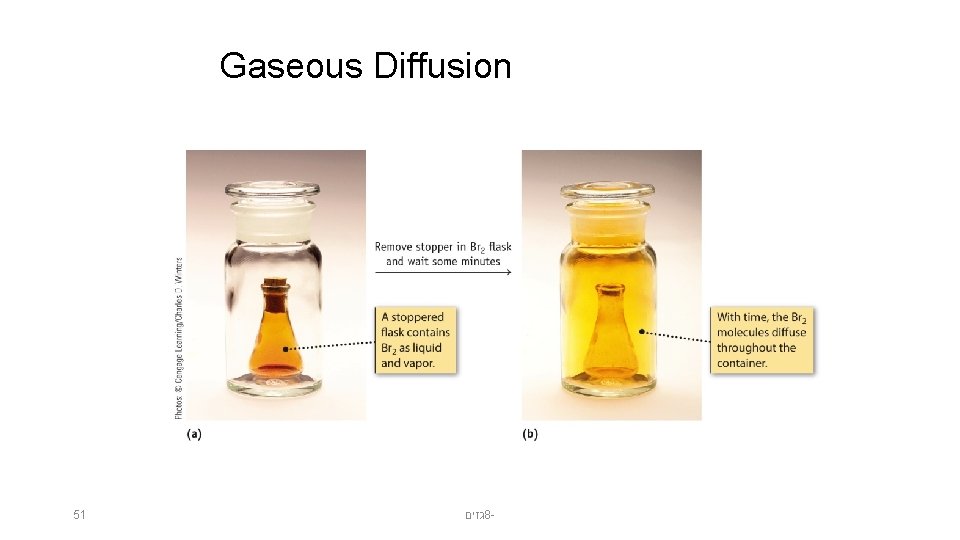
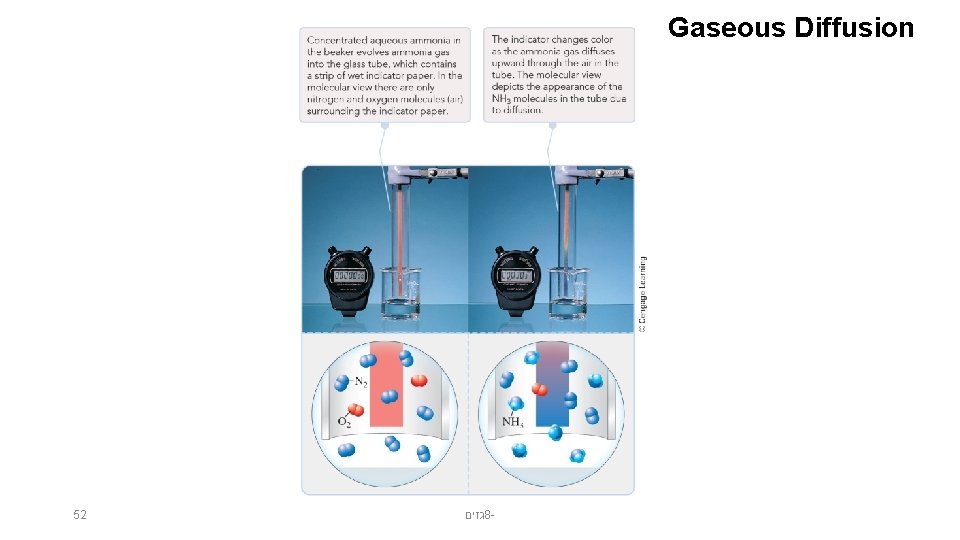
Gaseous Diffusion 51 גזים 8 -

Gaseous Diffusion 52 גזים 8 -

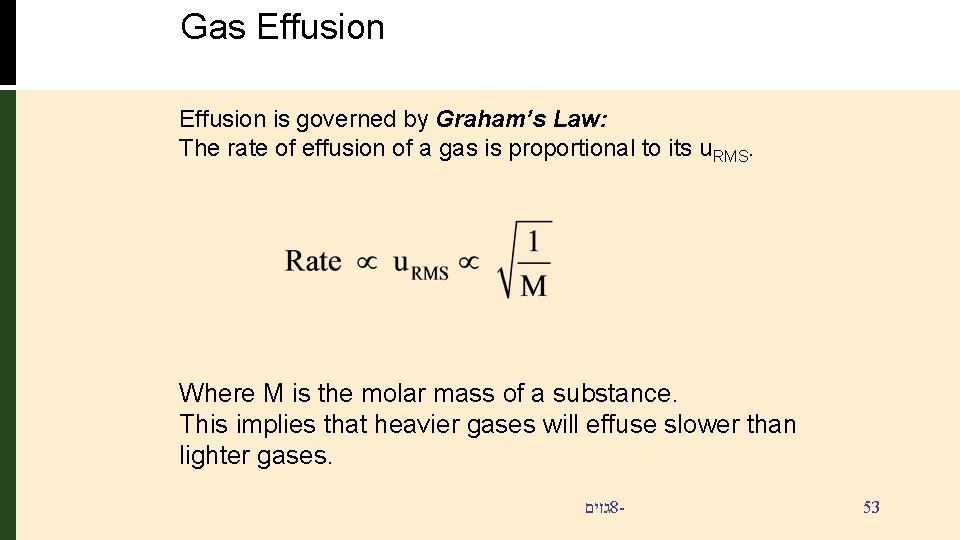
Gas Effusion is governed by Graham’s Law: The rate of effusion of a gas is proportional to its u. RMS. Where M is the molar mass of a substance. This implies that heavier gases will effuse slower than lighter gases. גזים 8 - 53

Graham’s Law of Effusion גזים 8 - 54

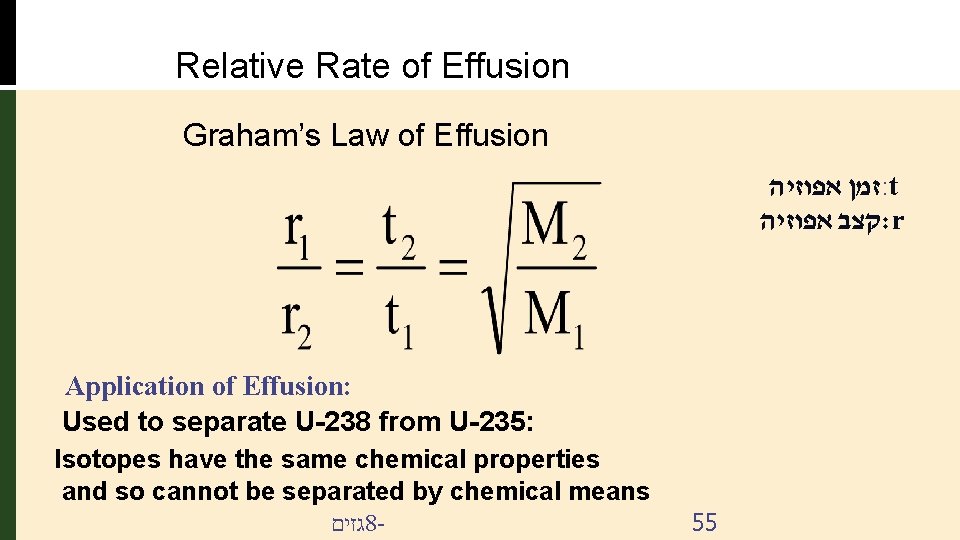
Relative Rate of Effusion Graham’s Law of Effusion זמן אפוזיה : t קצב אפוזיה : r Application of Effusion: Used to separate U-238 from U-235: Isotopes have the same chemical properties and so cannot be separated by chemical means גזים 8 - 55

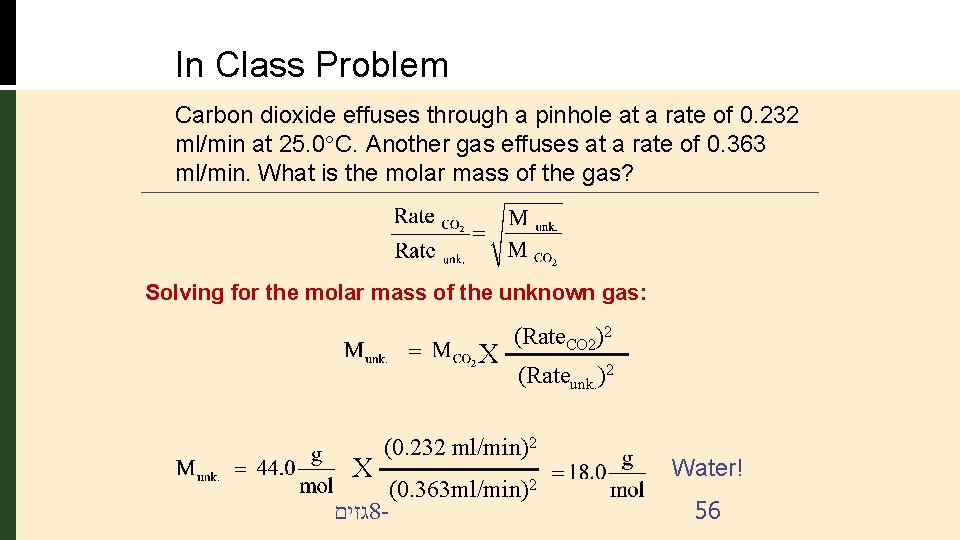
In Class Problem Carbon dioxide effuses through a pinhole at a rate of 0. 232 ml/min at 25. 0 C. Another gas effuses at a rate of 0. 363 ml/min. What is the molar mass of the gas? Solving for the molar mass of the unknown gas: X X (Rate. CO 2)2 (Rateunk. )2 (0. 232 ml/min)2 גזים 8 - (0. 363 ml/min)2 Water! 56
 Antigentest åre
Antigentest åre Physical properties of gases
Physical properties of gases Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Physical property and chemical property
Physical property and chemical property Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids What are the properties of solid
What are the properties of solid Properties of gas
Properties of gas Four properties of gas
Four properties of gas 5 properties of gases
5 properties of gases Properties of gases
Properties of gases The properties of noble gases
The properties of noble gases Gases have low densities
Gases have low densities Chemical properties of noble gases
Chemical properties of noble gases Properties of solid liquid and gas
Properties of solid liquid and gas State avogadro's law
State avogadro's law Properties of gases
Properties of gases List 2 of the important properties of gases
List 2 of the important properties of gases A gas occupies 473 cm3 at 36°c. find its volume at 94°c
A gas occupies 473 cm3 at 36°c. find its volume at 94°c Gases characteristics
Gases characteristics Characteristics of ideal gases
Characteristics of ideal gases Ideal gas law characteristics
Ideal gas law characteristics Descriptive matter
Descriptive matter Amino acid optical isomers
Amino acid optical isomers Properties of alkenes
Properties of alkenes Physical and chemical properties of sulphuric acid
Physical and chemical properties of sulphuric acid 2 hydrogen 1 oxygen
2 hydrogen 1 oxygen Ethan is observing chemical and physical properties
Ethan is observing chemical and physical properties 5 starch properties
5 starch properties Characteristics of pure water
Characteristics of pure water Thermal conductivity in dentistry
Thermal conductivity in dentistry Physical properties lesson 2
Physical properties lesson 2 Moreau
Moreau Chemical properties of air
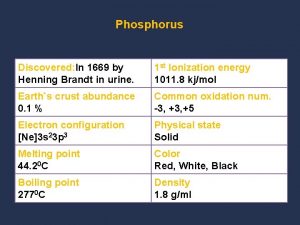
Chemical properties of air What are the physical properties of phosphorus
What are the physical properties of phosphorus Physical properties of paint
Physical properties of paint The physical properties of metals include luster and
The physical properties of metals include luster and Anything that has mass and takes up space is
Anything that has mass and takes up space is Thermal expansion of glass
Thermal expansion of glass Physical properties of protein
Physical properties of protein Physical properties of protein
Physical properties of protein Pumice crystal size
Pumice crystal size Yes or no
Yes or no Is brittleness a physical or chemical property
Is brittleness a physical or chemical property Naming amine
Naming amine Chemical properties of elements depend on
Chemical properties of elements depend on Chemical properties of helium
Chemical properties of helium Physical properties of solutions
Physical properties of solutions Physical properties of d-block elements
Physical properties of d-block elements Properties of aryl halides
Properties of aryl halides Properties of alkenes
Properties of alkenes Glowing splint
Glowing splint Propofol physical properties
Propofol physical properties Physical properties of magma
Physical properties of magma Derek bassett
Derek bassett Typical properties of polymers
Typical properties of polymers