Physical Properties Physical Properties Characteristics of a material









- Slides: 15

Physical Properties

Physical Properties • Characteristics of a material that can be observed or measured without changing the composition of the substances in the material.

• • Examples of Physical Properties Viscosity Conductivity Malleability Hardness Melting point Boiling point Density

Viscosity • The resistance of an object to flow. • Usually… – Thick liquids are more viscous and thin liquids are less viscous. – Heating a liquid makes it less viscous. • Which is more viscous, vinegar or corn syrup?

Conductivity • A material’s ability to allow heat/electricity to flow. • What types of materials have high conductivity? • What types of materials have low conductivity?

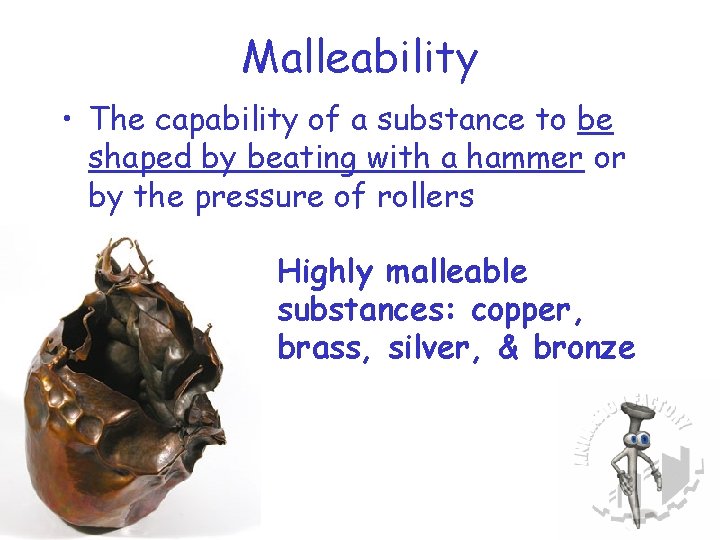
Malleability • The capability of a substance to be shaped by beating with a hammer or by the pressure of rollers Highly malleable substances: copper, brass, silver, & bronze

Hardness • The resistance of a metal to be scratched • For example, a kitchen knife can scratch a metal sheet of copper. – Stainless steel is harder than copper

Melting Point • The temperature at which a substance changes from a solid to a liquid. • Remember, the melting point of ice is O°C

Boiling Point • The temperature at which a liquid changes into a gas. • Remember, the boiling point of water is 100°C • Is the boiling point of a substance other than water the exact same as water’s boiling point?

Density • Every substance has a unique density, which can determine the purity of a substance. • The density of water is 1. 00 g/ml • The density of Sulfur is 2. 07 g/ml

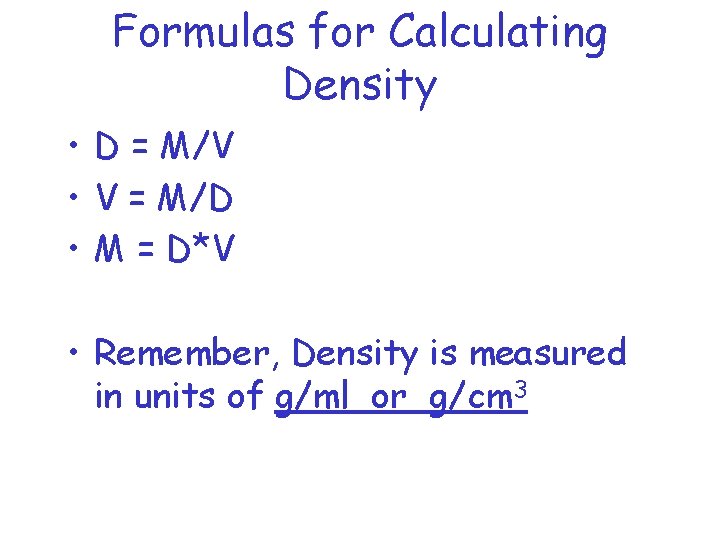
Formulas for Calculating Density • D = M/V • V = M/D • M = D*V • Remember, Density is measured in units of g/ml or g/cm 3

What can physical properties be used for? • Identify unknown materials • Separate substances in a mixture • Choose the best material suited to a specific purpose

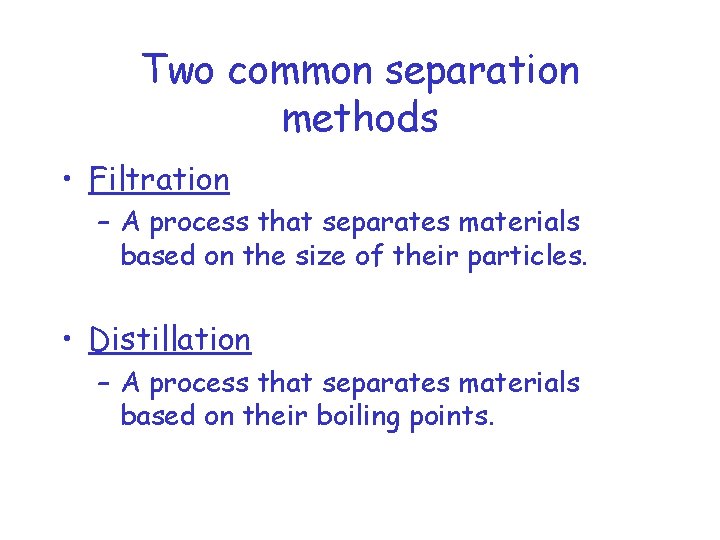
Two common separation methods • Filtration – A process that separates materials based on the size of their particles. • Distillation – A process that separates materials based on their boiling points.


Just Plain Fun • think about density
 Chemical properties of dental materials
Chemical properties of dental materials Is smell a physical property
Is smell a physical property Gd and t symbol
Gd and t symbol Standard costing and variance analysis formulas
Standard costing and variance analysis formulas Pop culture sports
Pop culture sports Non material culture examples
Non material culture examples Material and non material culture examples
Material and non material culture examples Examples of useful and harmful materials
Examples of useful and harmful materials Revision materials
Revision materials Physical properties of polymers
Physical properties of polymers Ideal properties of dental materials
Ideal properties of dental materials Material properties and definitions
Material properties and definitions Ideal root canal obturation
Ideal root canal obturation Glarlixi
Glarlixi Mechanical properties of cardboard
Mechanical properties of cardboard Reproducible evaluation of material properties
Reproducible evaluation of material properties