Chapter 6 Gases Physical Characteristics of Gases n









- Slides: 17

Chapter 6 - Gases

Physical Characteristics of Gases n Although gases have different chemical properties, gases have remarkably similar physical properties. ¨ Gases always fill their containers (recall solids and liquids). No definite shape and volume ¨ Gases are highly compressible: Volume decreases as pressure increases Volume increases as pressure decreases ¨ Gases diffuse (move spontaneously throughout any available space). ¨ Temperature affects either the volume or the pressure of a gas, or both.

Definition of a Gas n Therefore a definition for gas is: a substance that fills and assumes the shape of its container, diffuses rapidly, and mixes readily with other gases.

Three Gas Laws n Pressure ¨ force of colliding particles per unit area ¨ According to the KMT gases exert pressure due to the forces exerted by gas particles colliding with themselves and the sides of the container ¨ SI unit for pressure is kilopascals - k. Pa

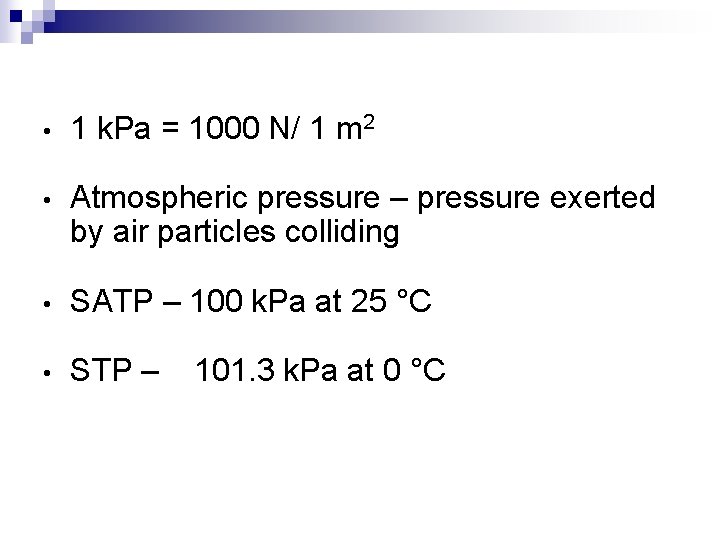
• 1 k. Pa = 1000 N/ 1 m 2 • Atmospheric pressure – pressure exerted by air particles colliding • SATP – 100 k. Pa at 25 °C • STP – 101. 3 k. Pa at 0 °C

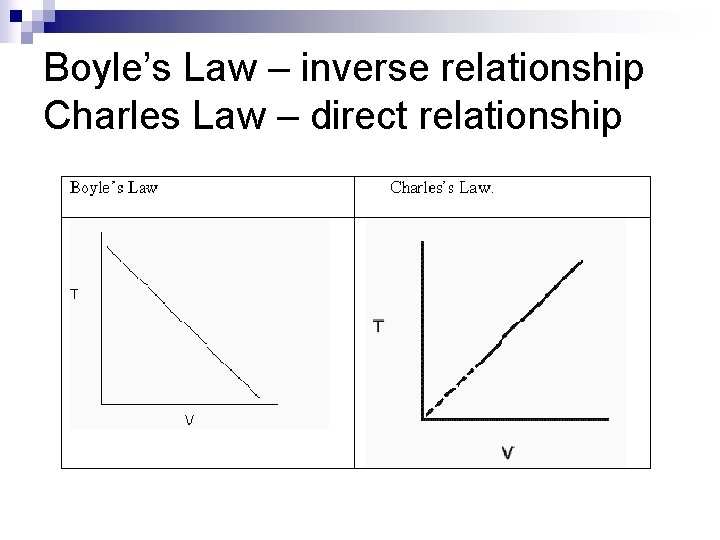
Boyle’s Law n n As pressure on a gas increases the volume of the gas decreases proportionally as the temperature is held constant P 1 V 1 = P 2 V 2

Charles Law n n the volume of a gas increases proportionally as the temperature of the gas increases, if the pressure is held Constant V 1 = V 2 T 1 T 2

Boyle’s Law – inverse relationship Charles Law – direct relationship

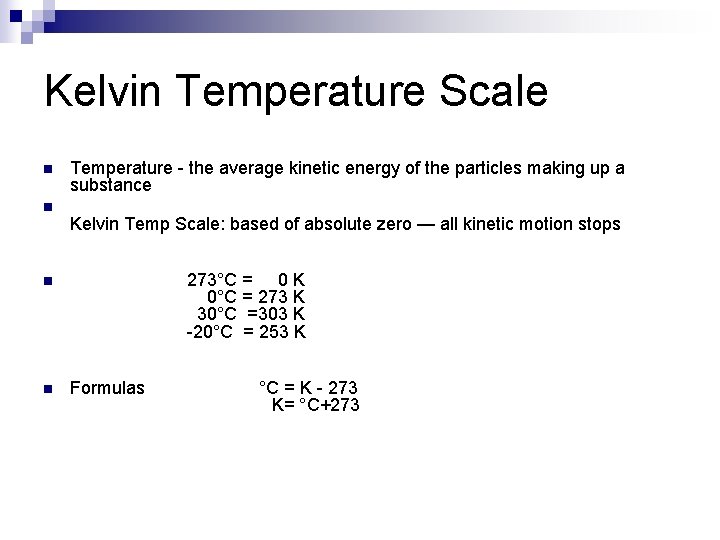
Kelvin Temperature Scale n n Temperature - the average kinetic energy of the particles making up a substance Kelvin Temp Scale: based of absolute zero — all kinetic motion stops 273°C = 0 K 0°C = 273 K 30°C =303 K -20°C = 253 K n n Formulas °C = K - 273 K= °C+273

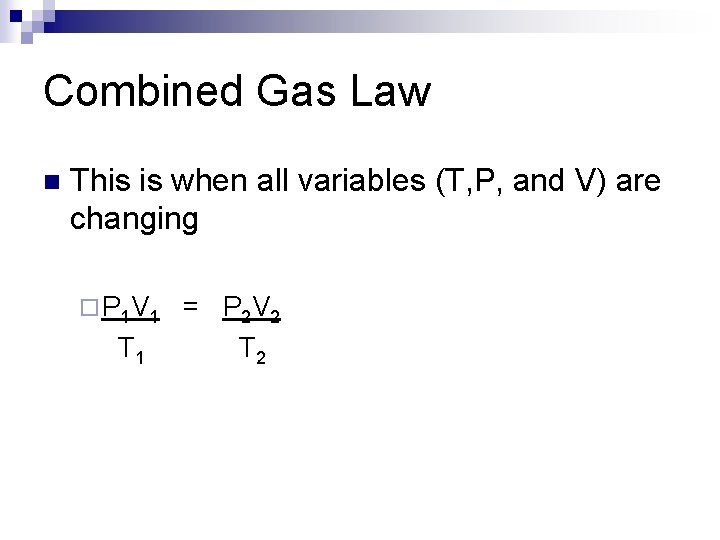
Combined Gas Law n This is when all variables (T, P, and V) are changing ¨ P 1 V 1 T 1 = P 2 V 2 T 2

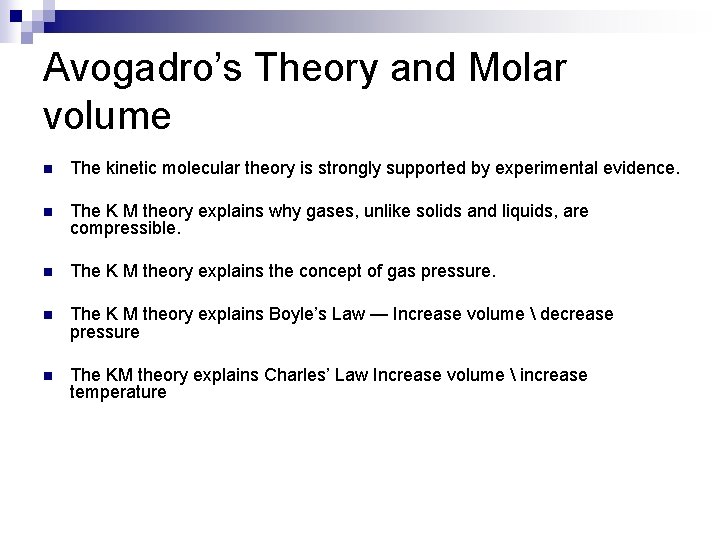
Avogadro’s Theory and Molar volume n The kinetic molecular theory is strongly supported by experimental evidence. n The K M theory explains why gases, unlike solids and liquids, are compressible. n The K M theory explains the concept of gas pressure. n The K M theory explains Boyle’s Law — Increase volume decrease pressure n The KM theory explains Charles’ Law Increase volume increase temperature

History Lesson n 1808 – Joseph Guy – Lussac ¨ “Law of Combining Volumes” n When measuring at the same temp and pressure, volumes of gas reactants and products (in chemical reactions) are always in simple whole number ratios n 1810 – Amadeo. Avogadro ¨ “Avogadro’s Theory” n Equal volumes of gases at the same temp and pressure have equal number of molecules

Molar Volume of Gases “new conversion ratio” n Avogadro says : § § T 1 = T 2 P 1 =P 2 V 1 = V 2 Then # particles of gas 1 = # particles of gas 2 1 mol = 6. 03 x 10 23 particles n Lets put these two ideas together…… n

Therefore for all gases at a specific temp and pressure there must be a certain volume that contains exactly 1 mole of particles - molar volume n The two most standard temps and pressures are STP and SATP n

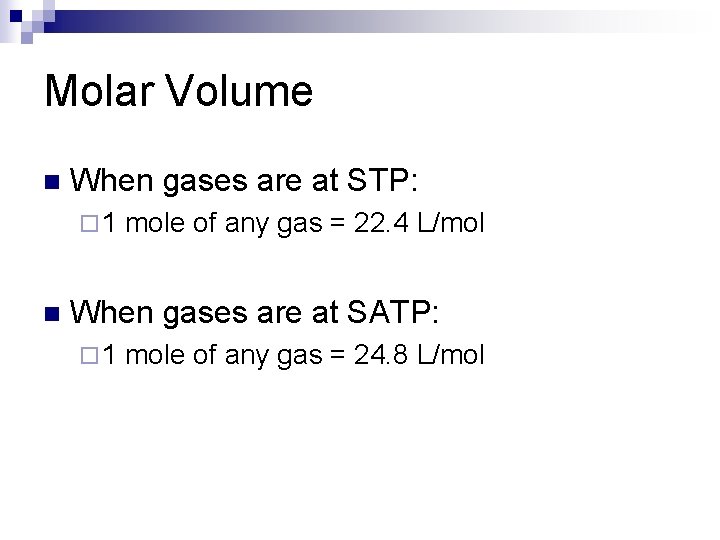
Molar Volume n When gases are at STP: ¨ 1 n mole of any gas = 22. 4 L/mol When gases are at SATP: ¨ 1 mole of any gas = 24. 8 L/mol

Ideal Gas Equation n Ideal Gas — is a hypothetical gas that obeys all the gas laws perfectly under all conditions. It is composed of particles with no attraction to each other. (Real gas particles do have a tiny attraction) n The further apart the gas particles are, the faster they are moving the less attractive force they have and behave the most like ideal gases n The smaller the molecules the closer the gas resembles an ideal gas n We assume ideal gases always. .

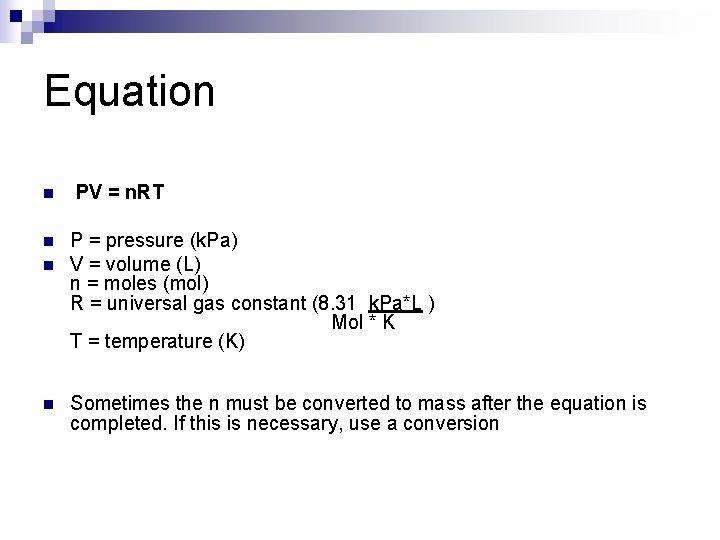
Equation n n PV = n. RT P = pressure (k. Pa) V = volume (L) n = moles (mol) R = universal gas constant (8. 31 k. Pa*L ) Mol * K T = temperature (K) Sometimes the n must be converted to mass after the equation is completed. If this is necessary, use a conversion
 Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Characteristics of gases
Characteristics of gases Characteristics of gases
Characteristics of gases Inert gases properties
Inert gases properties Gases characteristics
Gases characteristics Characteristics of ideal gases
Characteristics of ideal gases Ideal gases characteristics
Ideal gases characteristics Chapter 11 review gases section 1
Chapter 11 review gases section 1 Chapter 11 review gases section 1
Chapter 11 review gases section 1 Boyle's law variables
Boyle's law variables 14 the behavior of gases
14 the behavior of gases 13-4 practice problems chemistry
13-4 practice problems chemistry Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kinetic molecular theory of solid
Kinetic molecular theory of solid Pricing tripod in service marketing
Pricing tripod in service marketing What is physical fitness test in mapeh
What is physical fitness test in mapeh































