Chapter 13 Gases What Are Gases n Gases



















- Slides: 33

Chapter 13: Gases

What Are Gases? n Gases have mass n Much less compared to liquids and solids

Properties of Gases n Gases are easy to compress n Liquids and solids are difficult to compress

What Are Gases? n Gases fill their containers completely n Liquids and solids have fixed volumes

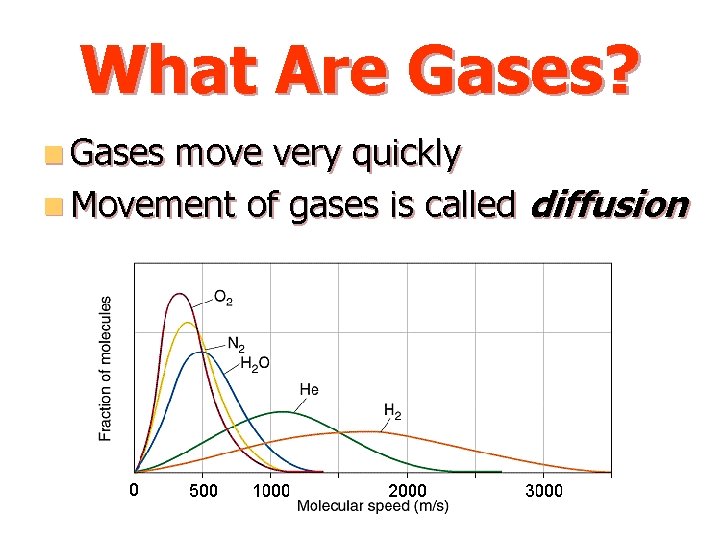
What Are Gases? n Gases move very quickly n Movement of gases is called diffusion

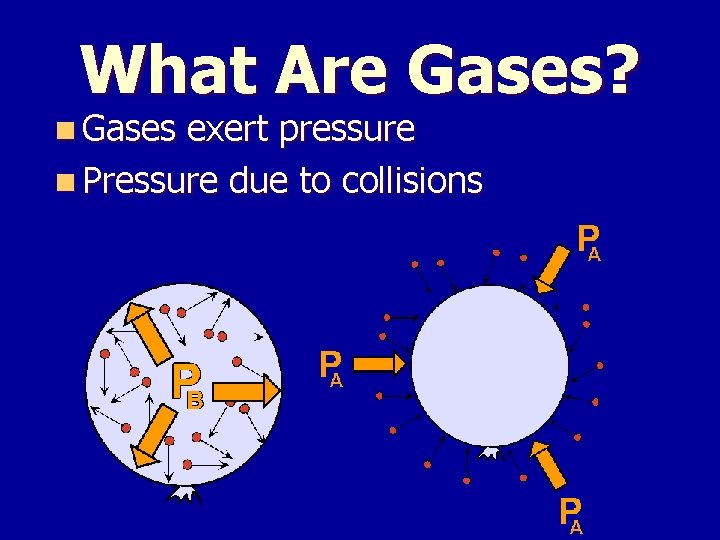
What Are Gases? n Gases exert pressure n Pressure due to collisions

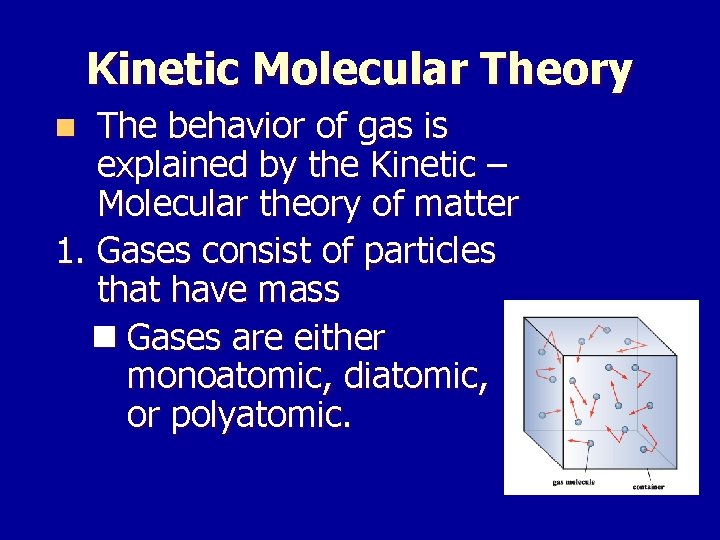
Kinetic Molecular Theory The behavior of gas is explained by the Kinetic – Molecular theory of matter 1. Gases consist of particles that have mass n Gases are either monoatomic, diatomic, or polyatomic. n

Kinetic Molecular Theory 2. There are large distances between gas particles – Volume of particles is assumed to be zero compared to the total volume 3. Gas particles are in constant random motion

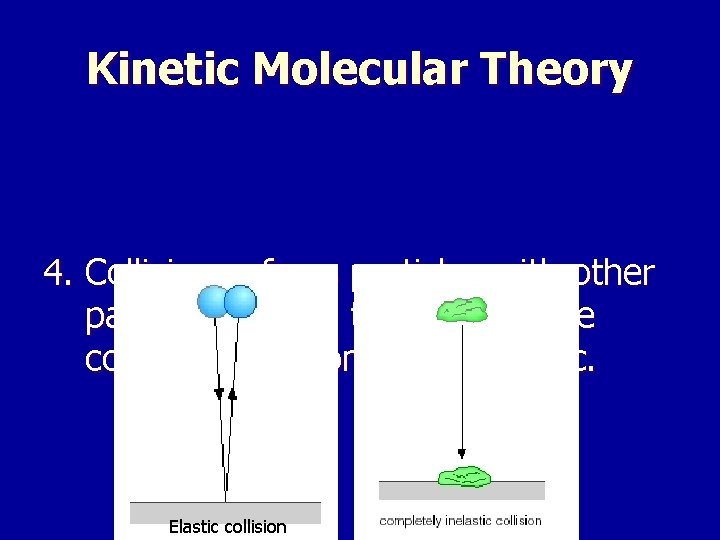
Kinetic Molecular Theory 4. Collisions of gas particles with other particles or with the walls of the container are completely elastic. Elastic collision

5. Kinetic energy of gas depends upon temperature Kinetic Molecular Theory – High temperature, high KE – Low temperature, low KE 6. Gas particles exert no attractive forces between one another www. falstad. com/gas/

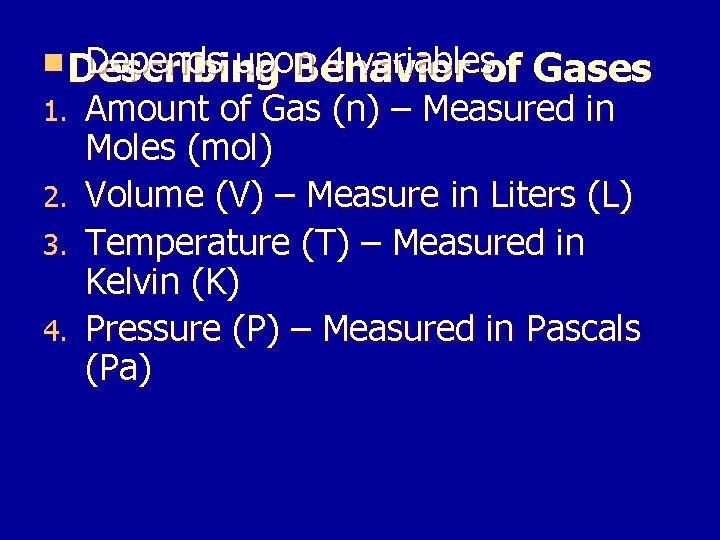
n Describing Depends upon 4 variablesof Behavior 1. 2. 3. 4. Gases Amount of Gas (n) – Measured in Moles (mol) Volume (V) – Measure in Liters (L) Temperature (T) – Measured in Kelvin (K) Pressure (P) – Measured in Pascals (Pa)

n Pressure is Pressure the amount of force per given area P = F/A n One newton of force per square meter is the Pascal n Often measure in k. Pa n

Atmospheric Pressure n Pressure exerted by the atmosphere

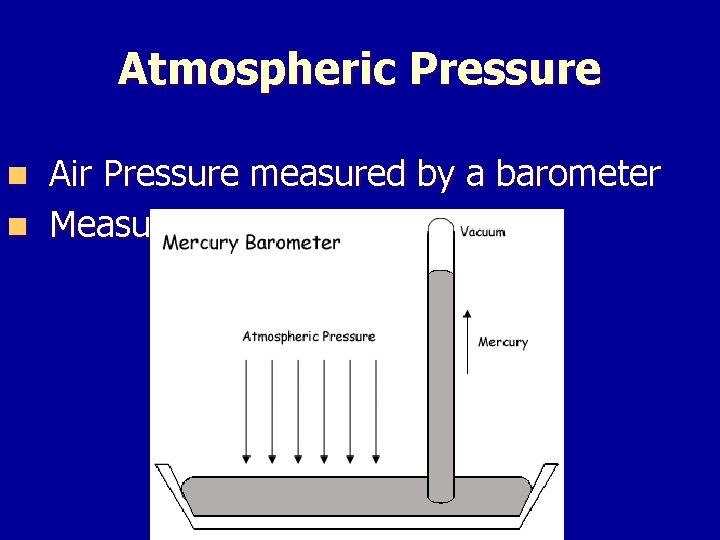
Atmospheric Pressure Air Pressure measured by a barometer n Measured in mm. Hg n

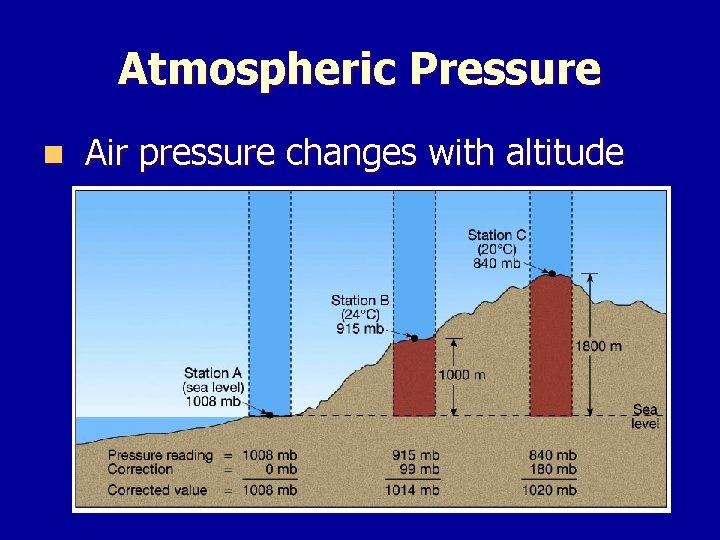
Atmospheric Pressure n Air pressure changes with altitude

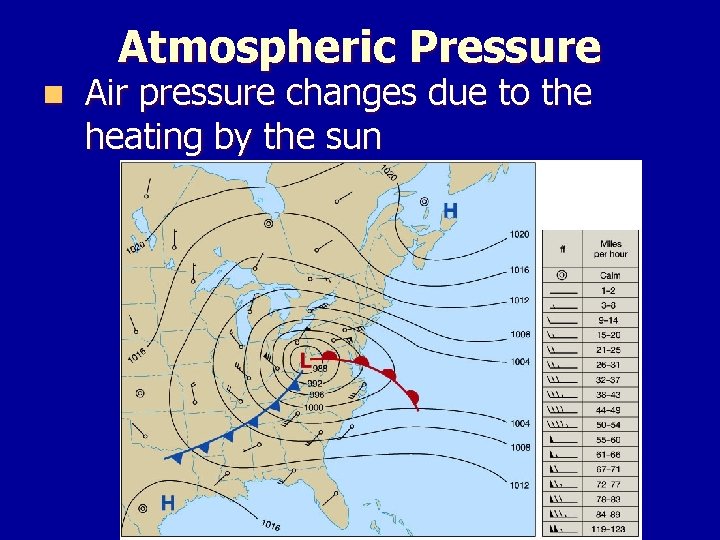
Atmospheric Pressure n Air pressure changes due to the heating by the sun

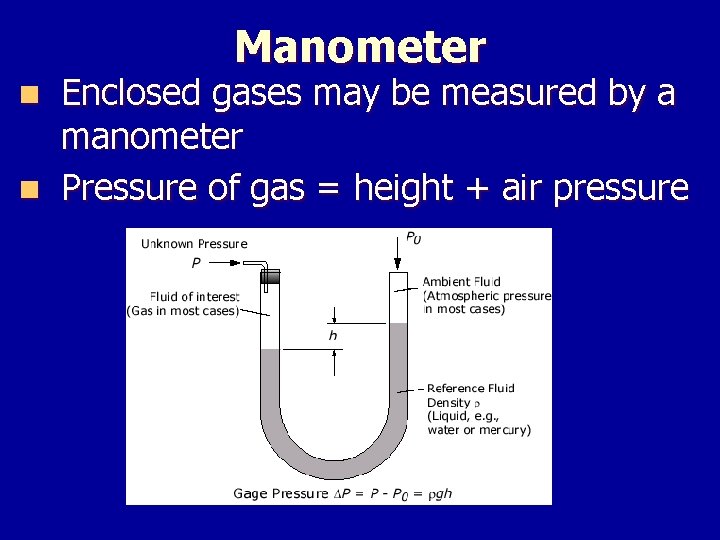
Manometer Enclosed gases may be measured by a manometer n Pressure of gas = height + air pressure n

Units of Pressure n 1013. 25 millibars (mb) = 101. 325 k. Pa = 1 atmosphere (atm) = 14. 7 pounds per in 2 (psi) = 760 mm of Hg (torr)

Practice n High Pressure 1085 mb in Mongolia (2001) Convert to k. Pa n Low Pressure 870 mb in a Pacific Typhoon (1979) Convert to Atm

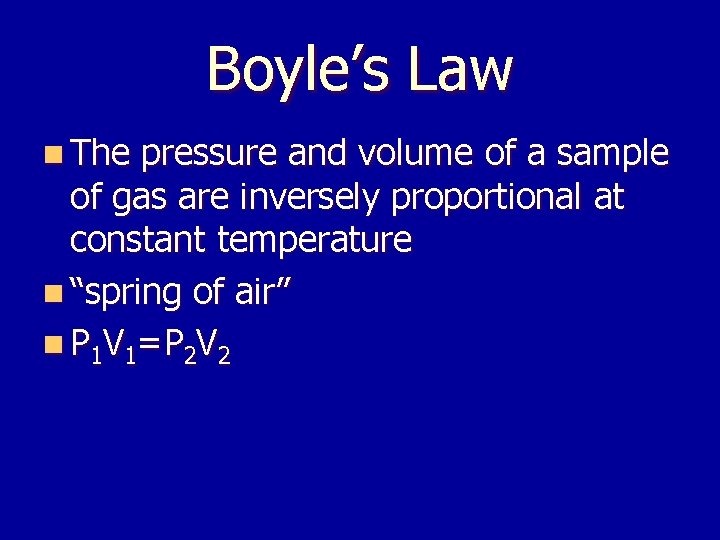
Boyle’s Law n The pressure and volume of a sample of gas are inversely proportional at constant temperature n “spring of air” n P 1 V 1=P 2 V 2


Practice n. A weather balloon contains 150. L of gas, internal gas pressure is 1. 0 atm. Atmospheric pressure at 41 km is 0. 4 atm. Calculate the new volume of the balloon. n 2. 00 L of a gas is at 740. 0 mm. Hg pressure. What is its volume at standard pressure?

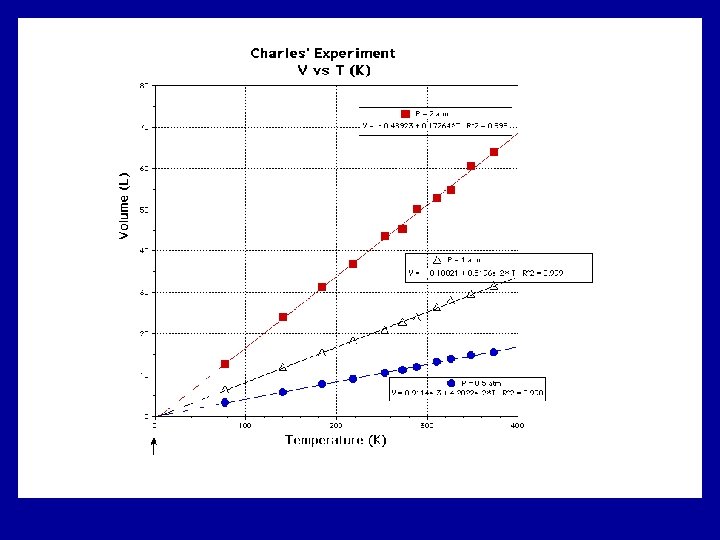
Charles’s Law n At constant pressure, the volume of a fixed amount of gas is directly proportional to its temperature. n Helped determine absolute zero n V 1/T 1=V 2/T 2 n All temperatures must be converted to Kelvin



Practice n What will be the volume of a gas sample at 355 K if its volume at 273 K is 8. 57 Liters? n At constant pressure a gas can be used as a thermometer. A sample of gas has a volume of 1 L at -18 0 C. What is the temperature if the volume of the same gas is changed to 0. 45 Liters?

Avogadro's Law n Avogadro’s Law states equal volumes of gases at the same temperature and pressure contain an equal number of particles. n Molar volume 22. 4 L at STP.

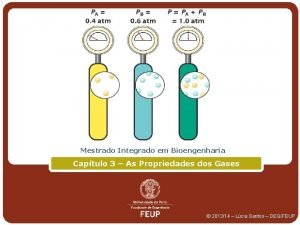
Dalton’s Law n Dalton’s Law of Partial Pressure states that the sum of the partial pressures of all of the components in a gas mixture is equal to the total pressure of the gas mixture. n PT = Pa + Pb + Pc ….


n What Practice is atmospheric pressure if the partial pressures of N 2, O 2, and Ar are 604. 5 mm Hg, 162. 8 mm Hg, and 0. 5 mm Hg respectively? n The gases of carbon dioxide, oxygen, nitrogen, neon, and krypton are mixed in a container. All gases have the same partial pressure and the total pressure is 33, 500 Pa. What is the partial pressure of nitrogen?

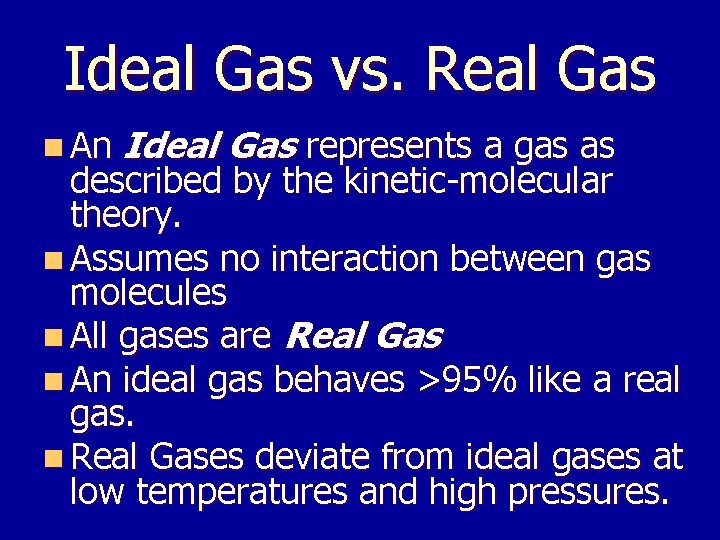
Ideal Gas vs. Real Gas n An Ideal Gas represents a gas as described by the kinetic-molecular theory. n Assumes no interaction between gas molecules n All gases are Real Gas n An ideal gas behaves >95% like a real gas. n Real Gases deviate from ideal gases at low temperatures and high pressures.

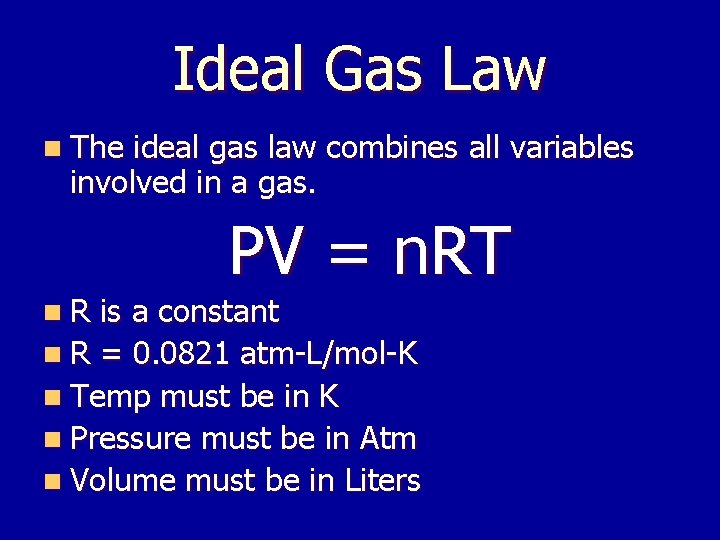
Ideal Gas Law n The ideal gas law combines all variables involved in a gas. PV = n. RT n. R is a constant n R = 0. 0821 atm-L/mol-K n Temp must be in K n Pressure must be in Atm n Volume must be in Liters

Practice n How many moles of a gas at 100 o. C does it take to fill a 1. 00 Liter flask to a pressure of 1. 50 atm? n What is the volume occupied by 9. 45 g of C 2 H 2 at STP? n How many kilograms of oxygen gas are contained in a sample that occupies 505 L at 675 k. Pa and -15 o. C?
 Antigentest åre
Antigentest åre Chapter 11 review gases section 1
Chapter 11 review gases section 1 Chapter 11 review gases section 1
Chapter 11 review gases section 1 Combined gas law practice worksheet answers
Combined gas law practice worksheet answers 14 the behavior of gases
14 the behavior of gases Chapter 13 gases
Chapter 13 gases Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Eficiencia de carnot
Eficiencia de carnot Boron
Boron Expansion of solids liquids and gases examples
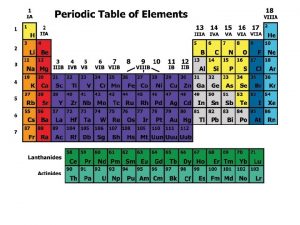
Expansion of solids liquids and gases examples Periodic table metals nonmetals metalloids noble gases
Periodic table metals nonmetals metalloids noble gases Gases on the periodic table
Gases on the periodic table Is the greenhouse effect good or bad
Is the greenhouse effect good or bad A gas occupies 473 cm3 at 36°c. find its volume at 94°c
A gas occupies 473 cm3 at 36°c. find its volume at 94°c The atmosphere is a mixture of
The atmosphere is a mixture of Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Gas stoichiometry
Gas stoichiometry Venn diagram solid liquid gas
Venn diagram solid liquid gas Gas exchange in circulatory system
Gas exchange in circulatory system Degree of freedom of gases
Degree of freedom of gases Equação geral dos gases
Equação geral dos gases Equação dos gases ideais
Equação dos gases ideais Gases have low densities
Gases have low densities Diffusion of gases across respiratory membranes:
Diffusion of gases across respiratory membranes: Mendeleev
Mendeleev Noble gas block
Noble gas block Fraccion molar gases
Fraccion molar gases Variables que afectan el comportamiento de los gases
Variables que afectan el comportamiento de los gases Constante dos gases perfeitos
Constante dos gases perfeitos Properties of a solid
Properties of a solid Comprensión de los gases
Comprensión de los gases Globo aerostatico ley de los gases
Globo aerostatico ley de los gases Constante r de los gases
Constante r de los gases