What is Matter Matter is anything that takes








- Slides: 11

What is Matter? Ø Matter is anything that takes up space and has mass. Ø Mass is the amount of matter in an object. 1

Properties Ø Words that describe matter (adjectives) Ø Physical Properties- a property that can be observed and measured without changing the substance. Ø Chemical Properties- a property that can only be observed by changing the type of substance. 2

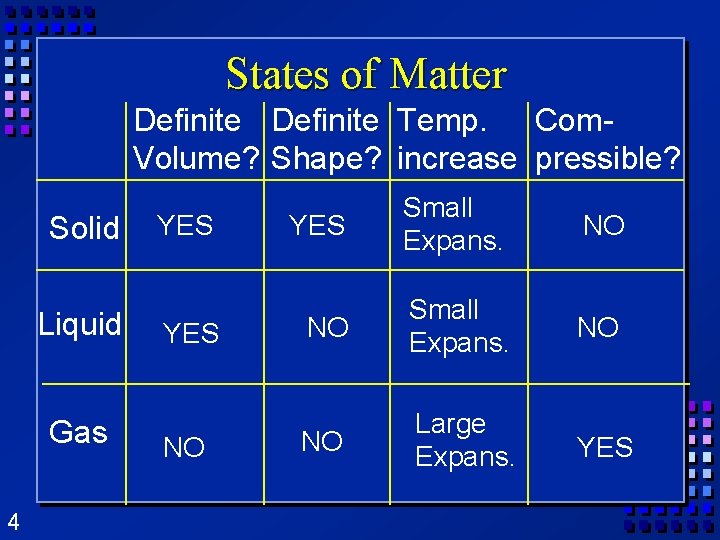
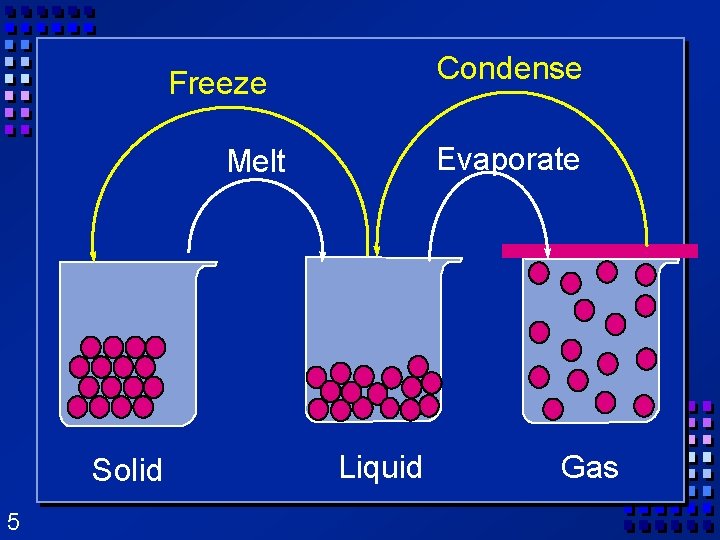
States of matter Ø Solid- mater that can not flow and has definite volume. Ø Liquid- definite volume but takes the shape of its container (flows). Ø Gas- a substance without definite volume or shape and can flow. 3

States of Matter Definite Temp. Com. Volume? Shape? increase pressible? Solid Liquid Gas 4 YES NO YES Small Expans. NO NO Large Expans. YES

Condense Freeze Evaporate Melt Solid 5 Liquid Gas

Another Way to Change States Ø Pressure Ø For some substances it will turn solids to liquids Ø For others it will turn liquids to solids – Silly putty Ø Will turn gas to liquid– Compressor in refrigerator and AC 6

Physical Changes ØA change that changes appearances, without changing the composition. Ø Examples? Ø Chemical changes - a change where a new form of matter is formed. Ø Also called chemical reaction. Ø Examples? Ø Not phase changes – Ice is still water. 7

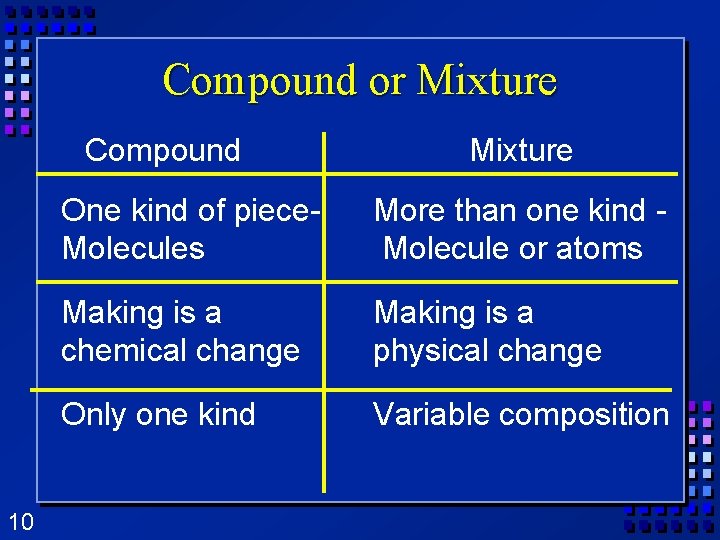
Ø Made Mixtures(rocks!!!) up of two substances. Ø Variable composition. Ø Heterogeneous- mixture is not the same from place to place. Ø Chocolate chip cookie, gravel, soil. Ø Homogeneous- same composition throughout. Ø Kool-aid, air. Ø Every part keeps its properties. 8

Ø Elements- Substances simplest kind of matter Ø Cannot be broken down into simpler Ø All one kind of atom. Ø Compounds (minerals!)are substances that can be broken down by chemical methods Ø When they are broken down, the pieces have completely different properties than the compound. Salt Ø Made of molecules- two or more atoms stuck together (minerals!!) 9

Compound or Mixture Compound 10 Mixture One kind of piece. Molecules More than one kind Molecule or atoms Making is a chemical change Making is a physical change Only one kind Variable composition

Chemical symbols Ø ESRT!! Names of elements on pg 16 Ø Subscripts tell us how many of each atom Ø H 2 O Ø C 3 H 8 Ø HBr. O 3 11
 Mass defintion
Mass defintion Is anything that has mass and takes up space.
Is anything that has mass and takes up space. Anything that takes up space
Anything that takes up space It is anything that occupies space and has mass.
It is anything that occupies space and has mass. Anything that takes up space and has mass
Anything that takes up space and has mass Anthing that takes up space and has mass is called?
Anthing that takes up space and has mass is called? Whats anything that has mass and takes up space
Whats anything that has mass and takes up space Mass vs matter
Mass vs matter A glass sinker has a mass m in air
A glass sinker has a mass m in air Anything that has mass and takes up space
Anything that has mass and takes up space It is anything that has mass and volume
It is anything that has mass and volume Thể thơ truyền thống
Thể thơ truyền thống


















