What is Matter Matter is anything that takes

















- Slides: 24

What is Matter? ö Matter is anything that takes up space and has mass. ö Mass- amount of material in an object w Everything has mass (even air) ö Weight is due to gravity, and changes from location to location; mass is always constant.

Kinetic Molecular Theory ö KMT w Particles of matter are always in motion. w causes thermal energy w The kinetic energy (speed) of these particles increases as temperature increases.

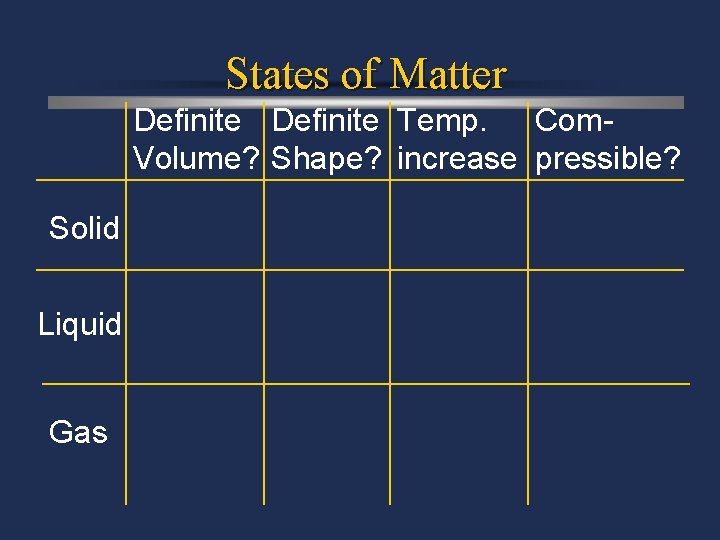
States of matter ö Solid- matter that can not flow (definite shape) and has definite volume. ö Liquid- definite volume but takes the shape of its container (flows). ö Gas- a substance without definite volume or shape and can flow.

States of Matter Definite Temp. Com. Volume? Shape? increase pressible? Solid Liquid Gas

B. Four States of Matter ö Solids w very low KE - particles vibrate but can’t move around w fixed shape w fixed volume

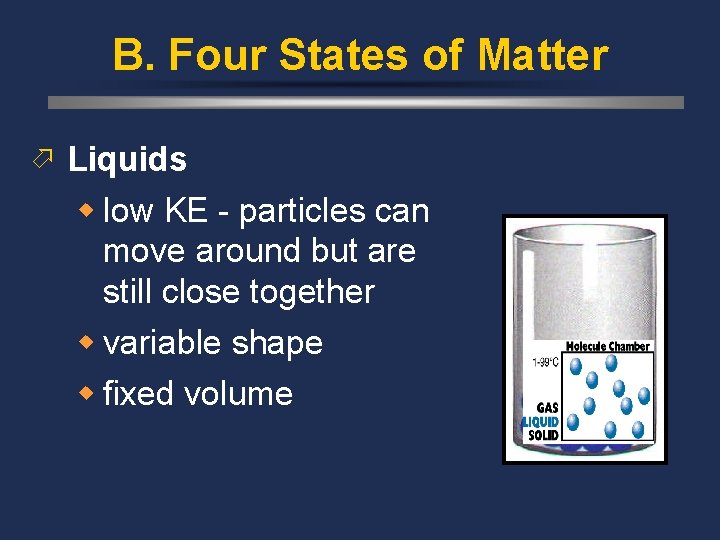
B. Four States of Matter ö Liquids w low KE - particles can move around but are still close together w variable shape w fixed volume

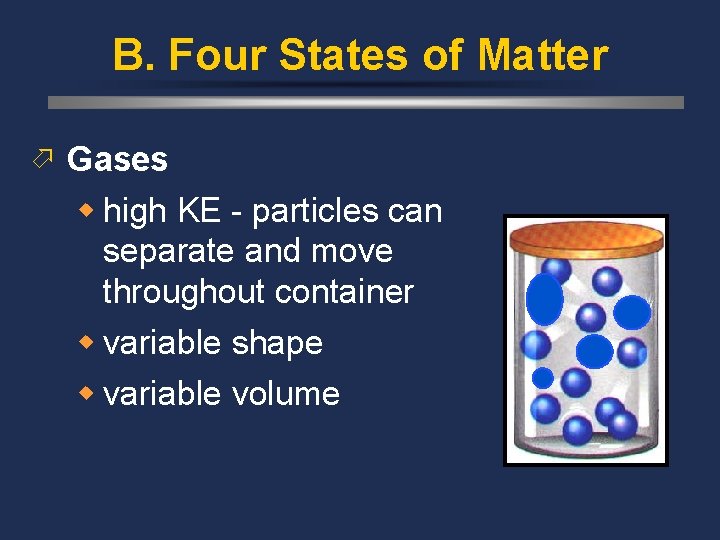
B. Four States of Matter ö Gases w high KE - particles can separate and move throughout container w variable shape w variable volume

B. Four States of Matter ö Plasma w very high KE - particles collide with enough energy to break into charged particles (+/-) w gas-like, variable shape & volume w stars, fluorescent light bulbs, CRTs

Types of Matter öMixture. Pure Substancea particular kind of more than one kind of matter; matter that iscomposition uniform (all the same) has a variable and has a definite composition. They are classified as elements & compounds w Water and gold

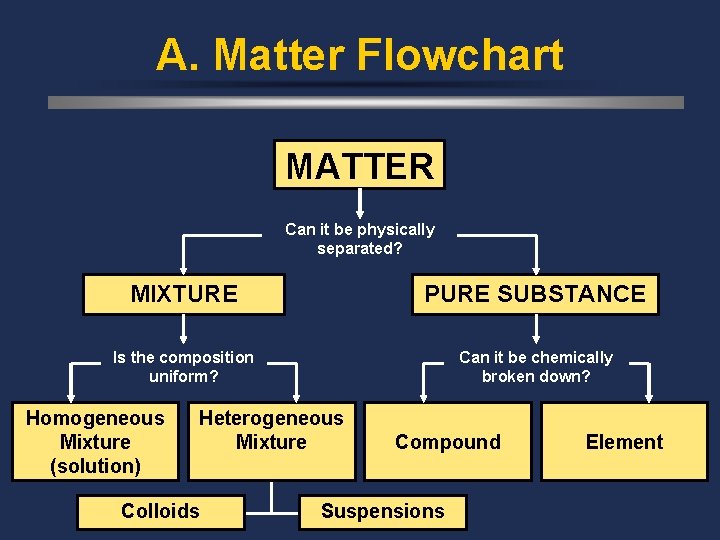
A. Matter Flowchart MATTER Can it be physically separated? MIXTURE PURE SUBSTANCE Is the composition uniform? Can it be chemically broken down? Homogeneous Mixture (solution) Heterogeneous Mixture Colloids Compound Suspensions Element

Matter Flowchart ö Examples: w graphite w pepper w sugar (sucrose) w paint w soda

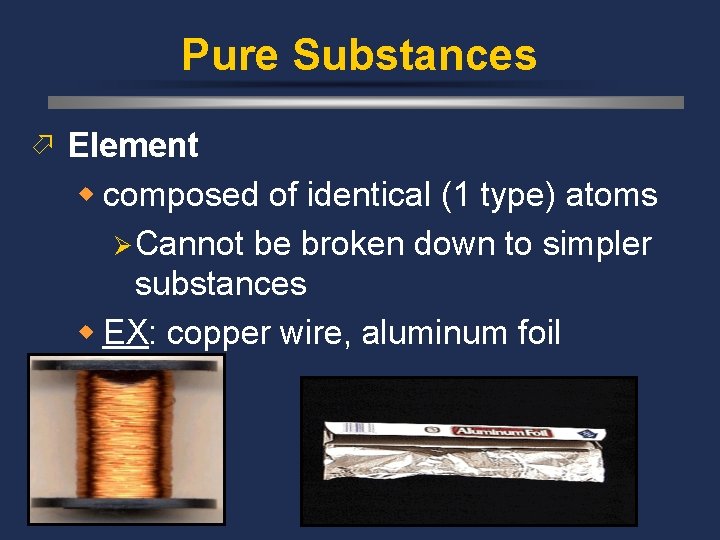
Pure Substances ö Element w composed of identical (1 type) atoms Ø Cannot be broken down to simpler substances w EX: copper wire, aluminum foil

Pure Substances ö Compound w composed of 2 or more elements in a fixed ratio Ø Can be broken down by chemical means w properties differ from those of individual elements w EX: table salt (Na. Cl), sugar (C 6 H 12 O 6)

Pure Substances ö Law of Definite Composition w A given compound always contains the same, fixed ratio of elements. ö Law of Multiple Proportions w Elements can combine in different ratios to form different compounds.

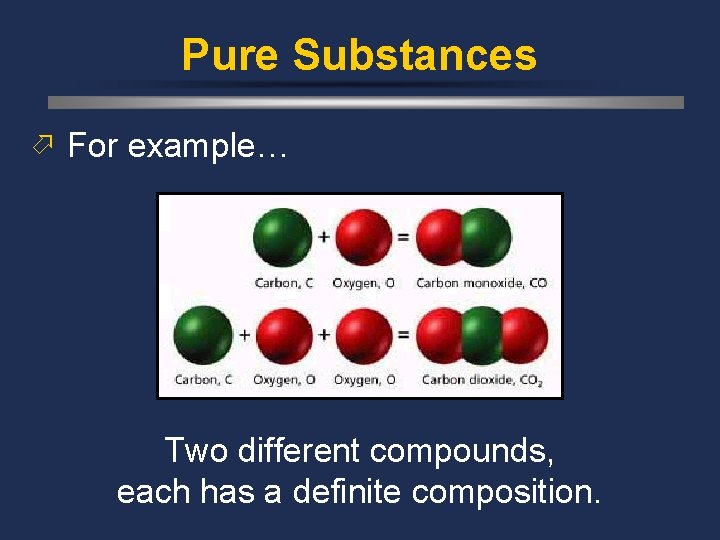
Pure Substances ö For example… Two different compounds, each has a definite composition.

Mixtures ö Can be separated into at least two pure substances; variable composition. Every part keeps it’s own properties. w Heterogeneous- mixture is not uniform in composition Ø Chocolate chip cookie, gravel, soil. w Homogeneous- same composition throughout; called “solutions” Ø Kool-aid, chocolate milk, salt water

Mixtures ö Variable combination of 2 or more pure substances.

Mixtures ö Solution w Homogeneous Ø Uniform in appearance Tyndall Effect w very small particles w no Tyndall effect w particles don’t settle w 1 phase present at a time w EX: rubbing alcohol

Solutions Continued ö Mixed molecule by molecule ö Can occur between any state of matter w gas in gas; liquid in gas; gas in liquid; solid in solid (alloys), etc.

Solutions continued ö Like all mixtures, they keep the properties of the components. ö Some can be separated easily by physical means: rocks and marbles, iron filings and sulfur ö Other methods: distillationtakes advantage of different boiling points (physical properties)

Mixtures ö Colloid w heterogeneous w medium-sized particles w Tyndall effect w particles don’t settle w Variable composition w 2 or more physically distinct phases may be present w EX: milk

Mixtures ö Suspension w heterogeneous w large particles w Tyndall effect w particles settle w Variable composition w 2 or more physically distinct phases may be present w EX: fresh-squeezed lemonade

Mixtures ö Examples: w mayonnaise w muddy water w fog w saltwater w Italian salad dressing

Compound or Mixture Compound Mixture
 Anything that occupies space and has mass what is it
Anything that occupies space and has mass what is it Defintion of mass
Defintion of mass Matter is anything that has and takes up
Matter is anything that has and takes up Matter is anything that takes up space and has
Matter is anything that takes up space and has Matter anything that
Matter anything that Anything that has mass and volume
Anything that has mass and volume Anything that has mass and takes up space
Anything that has mass and takes up space Anthing that takes up space and has mass is called?
Anthing that takes up space and has mass is called? Matter is anything that has
Matter is anything that has Matter is anything that has mass and
Matter is anything that has mass and All matter has and takes up
All matter has and takes up Block nhĩ thất cao độ
Block nhĩ thất cao độ Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Tôn thất thuyết là ai
Tôn thất thuyết là ai Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Matter anything that
Matter anything that Matter anything that
Matter anything that Dmv formula
Dmv formula Matter is anything that:
Matter is anything that: