Matter Matter is anything that takes up space

















- Slides: 31


Matter

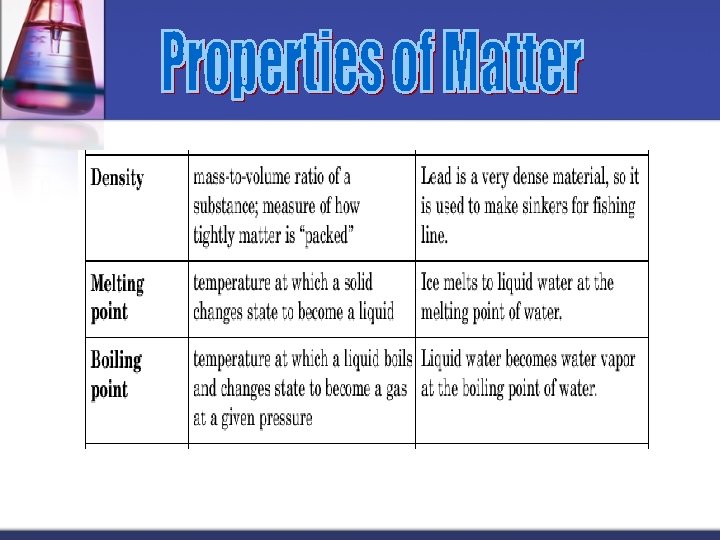
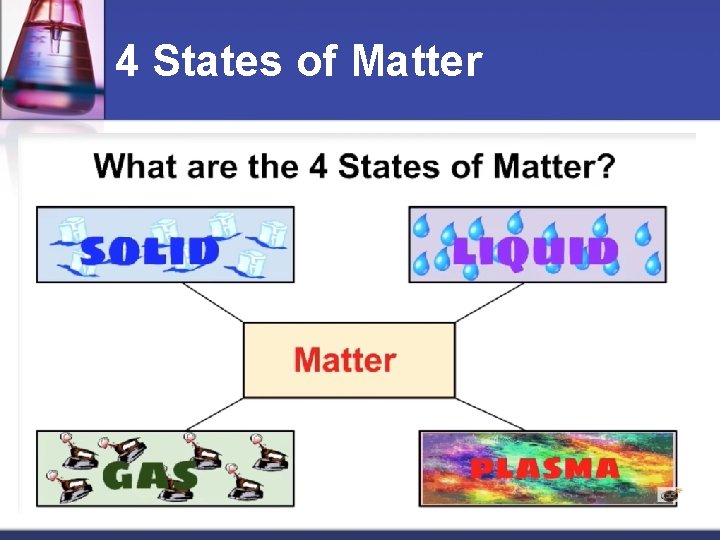
Matter is anything that takes up space & has mass. n It occurs in three states: n Solid n Liquid n Gas n


States of Matter


4 States of Matter

States of Matter

Solids are matter with a definite shape and volume. n The particles are in a tight, regular pattern. n The particles are close together and vibrate n A solid does not take the shape of a container in which it is placed. n


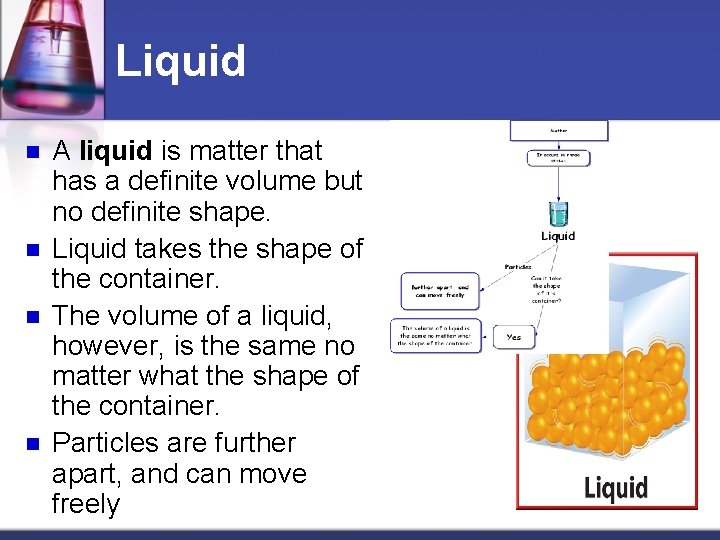
Liquid n n A liquid is matter that has a definite volume but no definite shape. Liquid takes the shape of the container. The volume of a liquid, however, is the same no matter what the shape of the container. Particles are further apart, and can move freely


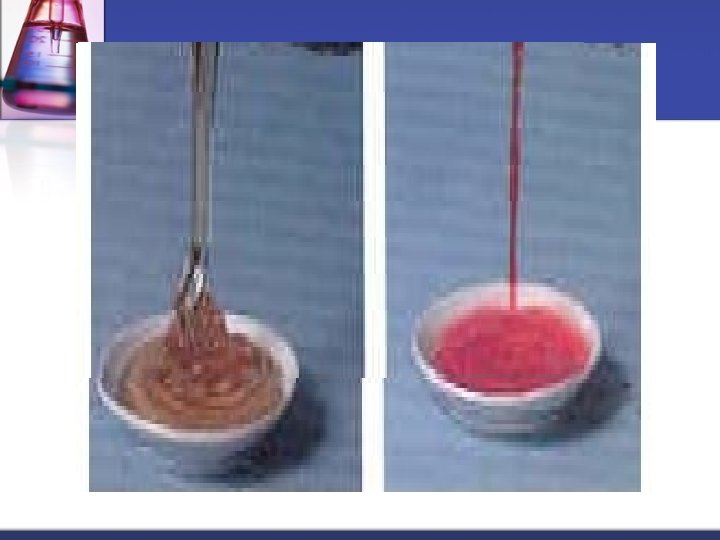
Viscosity A liquid’s resistance to flow is known as the liquid’s viscosity. n The slower a liquid flows, the higher its viscosity is. n For many liquids, viscosity increases as the liquid becomes colder. n We can see that the honey is much more viscous than the red solution picture from http: //www. physics. hku. hk/~phys 0607/lectures/chap 05. html


Surface Tension n n The uneven forces acting on the particles on the surface of a liquid are called surface tension. Surface tension causes the liquid to act as if a thin film were stretched across its surface.


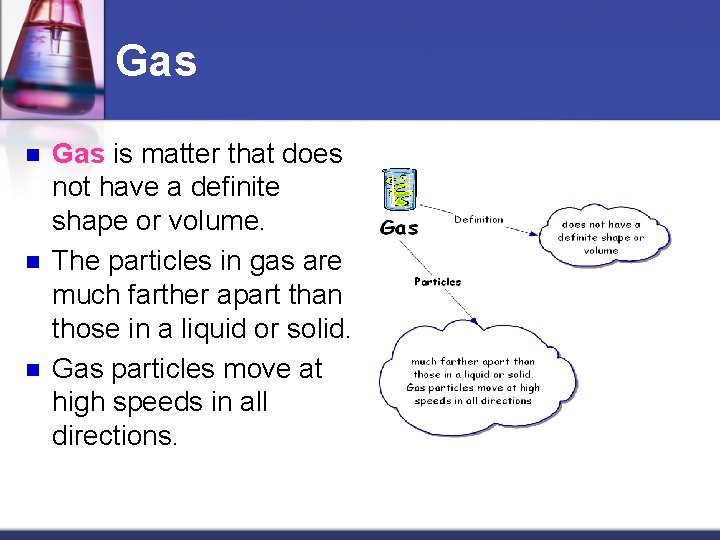
Gas n n n Gas is matter that does not have a definite shape or volume. The particles in gas are much farther apart than those in a liquid or solid. Gas particles move at high speeds in all directions.


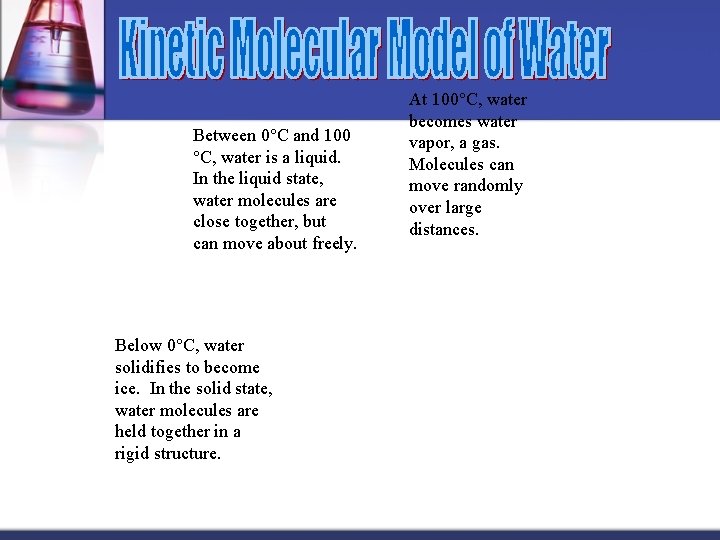
Between 0°C and 100 °C, water is a liquid. In the liquid state, water molecules are close together, but can move about freely. Below 0°C, water solidifies to become ice. In the solid state, water molecules are held together in a rigid structure. At 100°C, water becomes water vapor, a gas. Molecules can move randomly over large distances.

Changes in State of Matter

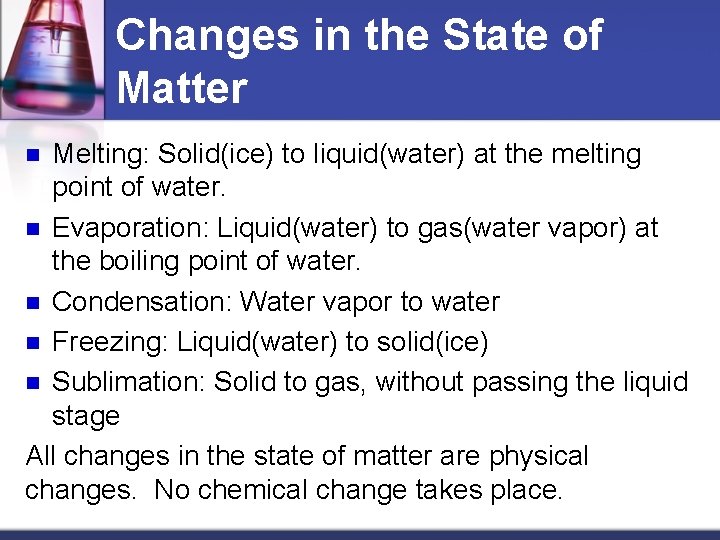
Changes in the State of Matter Melting: Solid(ice) to liquid(water) at the melting point of water. n Evaporation: Liquid(water) to gas(water vapor) at the boiling point of water. n Condensation: Water vapor to water n Freezing: Liquid(water) to solid(ice) n Sublimation: Solid to gas, without passing the liquid stage All changes in the state of matter are physical changes. No chemical change takes place. n

States of Matter n https: //phet. colorado. edu/sims/html/states-ofmatter/latest/states-of-matter_en. html

2. In which of the following are the particles closest together? A. Solid B. Liquid C. Gas D. Fluid

All matter, regardless of state, undergoes physical and chemical changes. These changes can be microscopic or macroscopic.


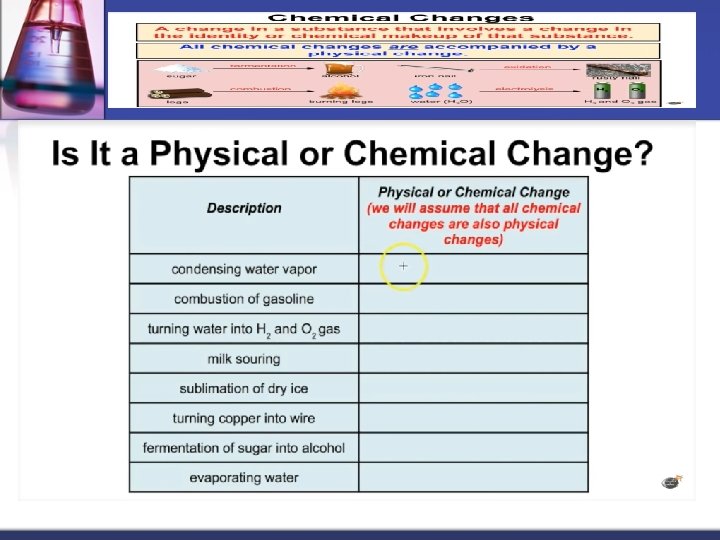
A physical change occurs when the substance changes state but does not change its chemical composition. Can be observed or measured. For example: water freezing into ice, cutting a piece of wood into smaller pieces, etc. The form or appearance has changed, but the properties of that substance are the same ( it has the same melting point, boiling point, chemical composition, etc. )

n n n Melting point Boiling point Vapor pressure Color State of matter n n n Density Electrical conductivity Solubility Adsorption to a surface Hardness


A chemical change occurs when a substance changes into something new. This occurs due to heating, chemical reaction, etc. You can tell a chemical change has occurred if the density, melting point or freezing point of the original substance changes. Many common signs of a chemical change can be seen (bubbles forming, mass changed, etc).

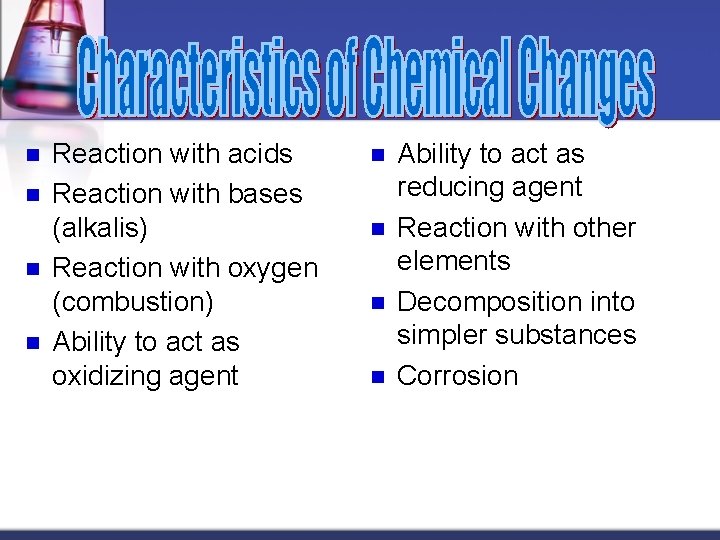
n n Reaction with acids Reaction with bases (alkalis) Reaction with oxygen (combustion) Ability to act as oxidizing agent n n Ability to act as reducing agent Reaction with other elements Decomposition into simpler substances Corrosion
