HONORS What is Matter Matter anything that takes

- Slides: 13

HONORS

What is Matter? • Matter = anything that takes up space and has mass – Matter is either a substance or a mixture • Substance - all particles in matter are identical (fixed composition) • Mixture – two or more substances physically combined (variable composition)

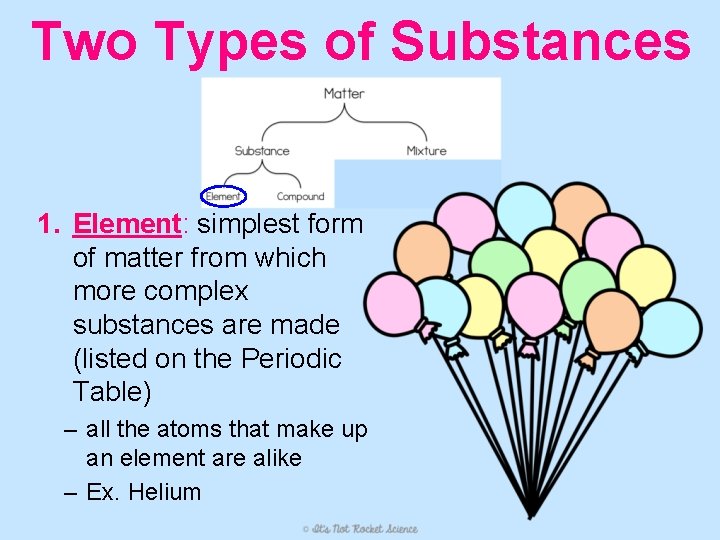
Two Types of Substances 1. Element: simplest form of matter from which more complex substances are made (listed on the Periodic Table) – all the atoms that make up an element are alike – Ex. Helium

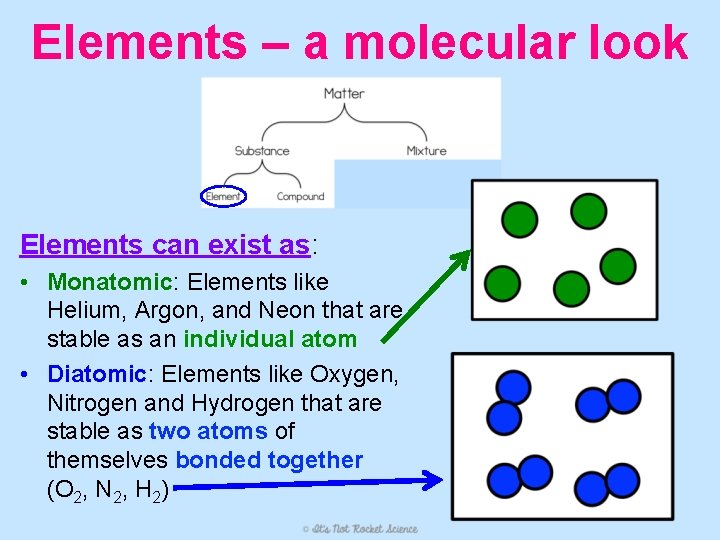
Elements – a molecular look Elements can exist as: • Monatomic: Elements like Helium, Argon, and Neon that are stable as an individual atom • Diatomic: Elements like Oxygen, Nitrogen and Hydrogen that are stable as two atoms of themselves bonded together (O 2, N 2, H 2)

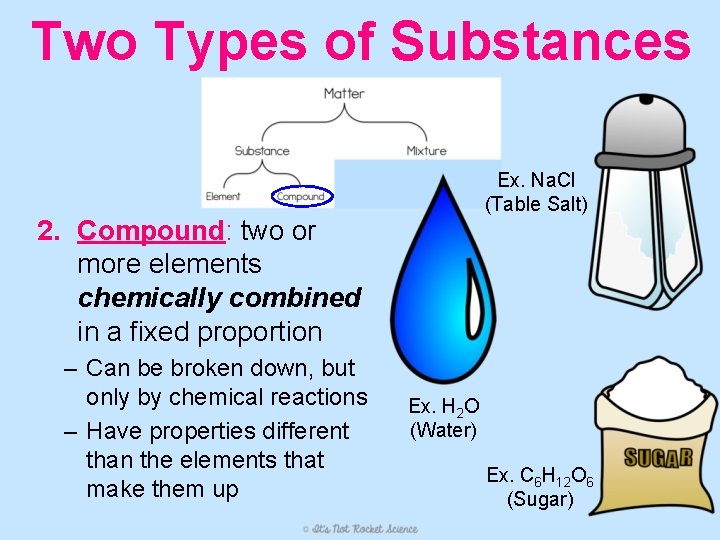
Two Types of Substances Ex. Na. Cl (Table Salt) 2. Compound: two or more elements chemically combined in a fixed proportion – Can be broken down, but only by chemical reactions – Have properties different than the elements that make them up Ex. H 2 O (Water) Ex. C 6 H 12 O 6 (Sugar)

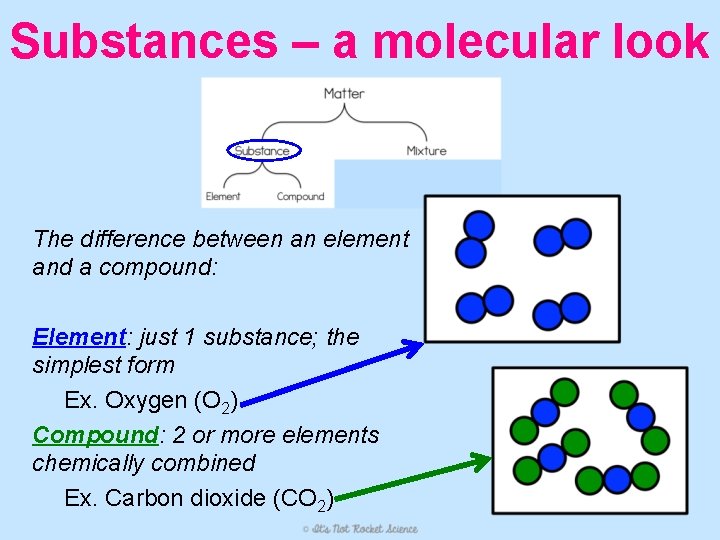
Substances – a molecular look The difference between an element and a compound: Element: just 1 substance; the simplest form Ex. Oxygen (O 2) Compound: 2 or more elements chemically combined Ex. Carbon dioxide (CO 2)

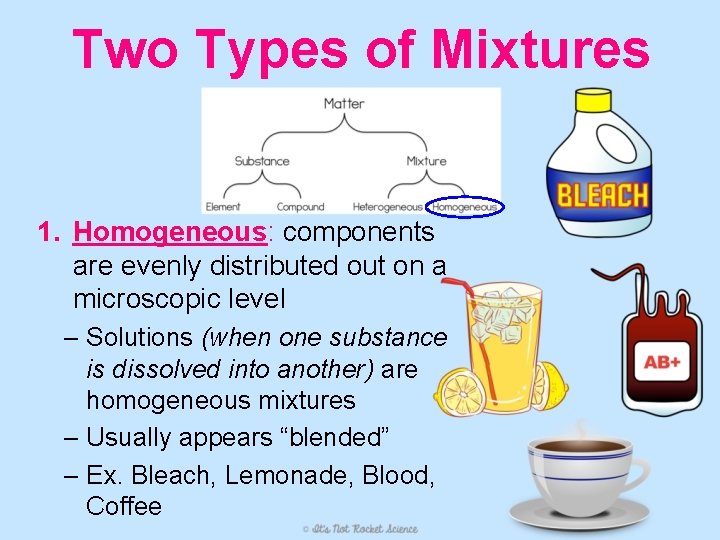
Two Types of Mixtures 1. Homogeneous: components are evenly distributed out on a microscopic level – Solutions (when one substance is dissolved into another) are homogeneous mixtures – Usually appears “blended” – Ex. Bleach, Lemonade, Blood, Coffee

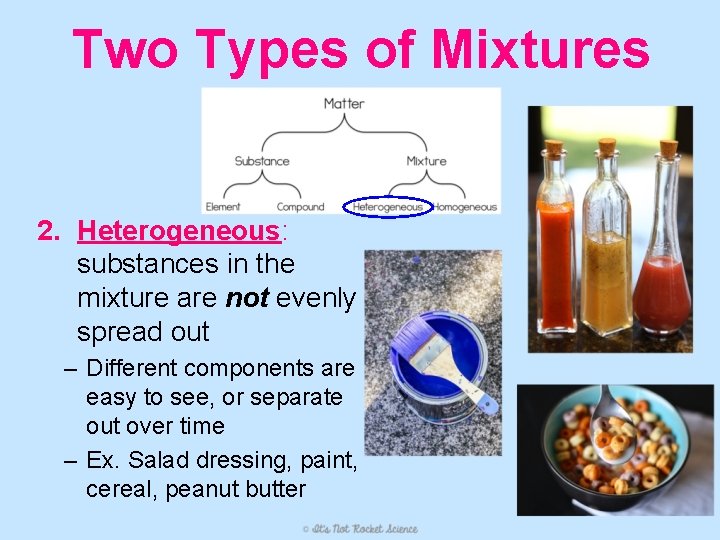
Two Types of Mixtures 2. Heterogeneous: substances in the mixture are not evenly spread out – Different components are easy to see, or separate out over time – Ex. Salad dressing, paint, cereal, peanut butter

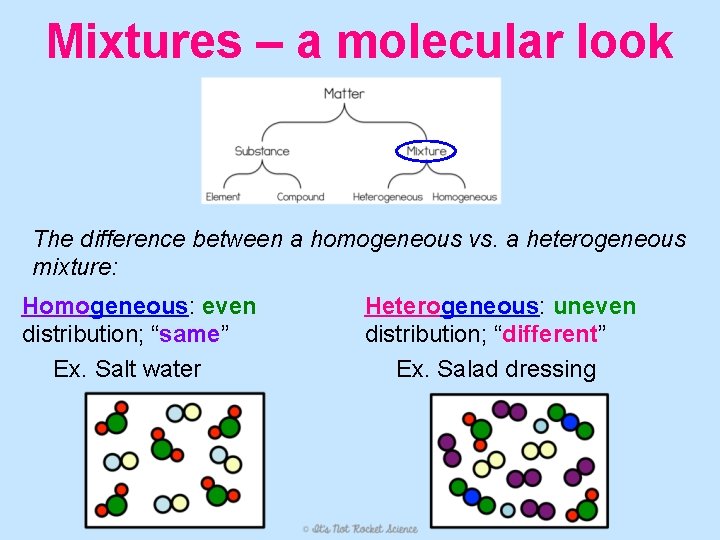
Mixtures – a molecular look The difference between a homogeneous vs. a heterogeneous mixture: Homogeneous: even distribution; “same” Ex. Salt water Heterogeneous: uneven distribution; “different” Ex. Salad dressing

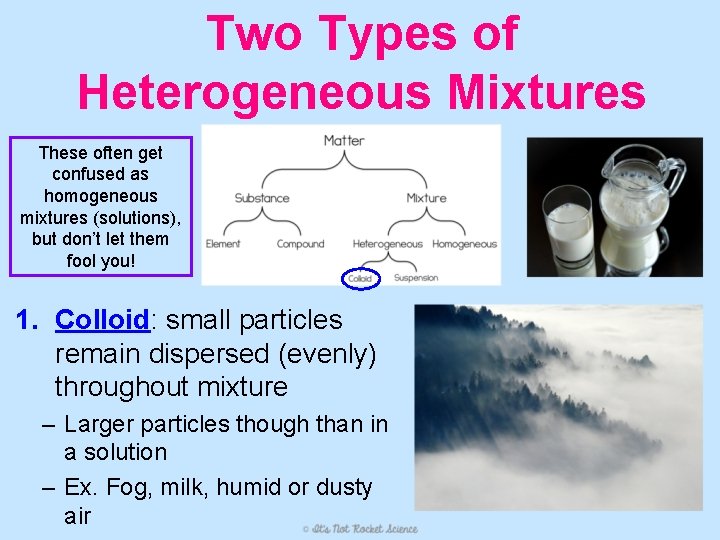
Two Types of Heterogeneous Mixtures These often get confused as homogeneous mixtures (solutions), but don’t let them fool you! 1. Colloid: small particles remain dispersed (evenly) throughout mixture – Larger particles though than in a solution – Ex. Fog, milk, humid or dusty air

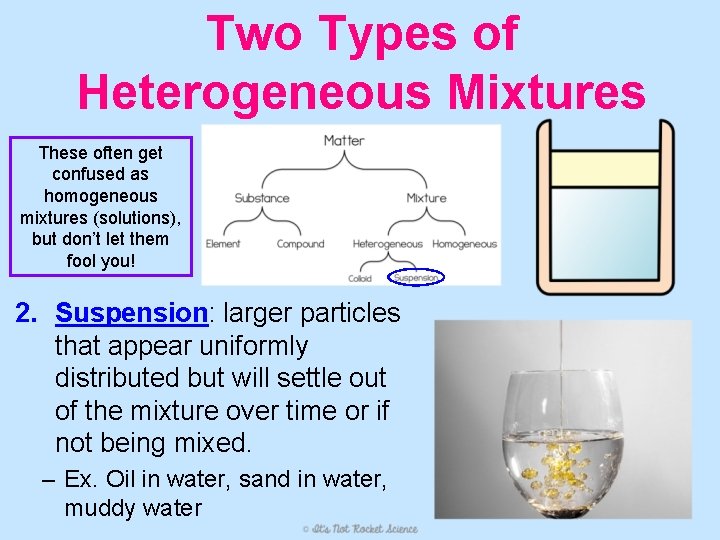
Two Types of Heterogeneous Mixtures These often get confused as homogeneous mixtures (solutions), but don’t let them fool you! 2. Suspension: larger particles that appear uniformly distributed but will settle out of the mixture over time or if not being mixed. – Ex. Oil in water, sand in water, muddy water

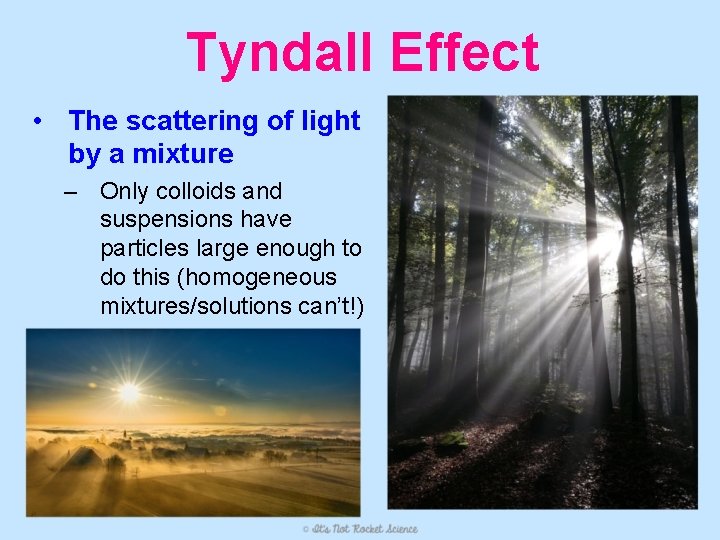
Tyndall Effect • The scattering of light by a mixture – Only colloids and suspensions have particles large enough to do this (homogeneous mixtures/solutions can’t!)

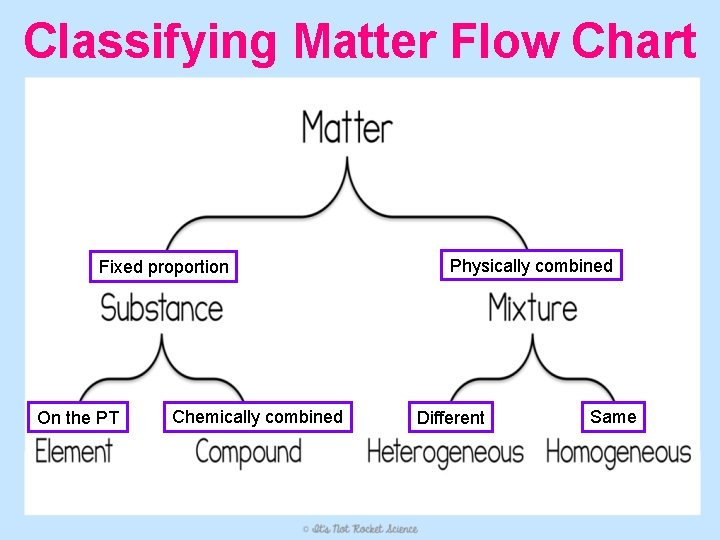
Classifying Matter Flow Chart Fixed proportion On the PT Chemically combined Physically combined Different Same
 Matter is anything that
Matter is anything that Matter is anything that has and takes up
Matter is anything that has and takes up All matter has and takes up
All matter has and takes up Anything that has mass and occupies space.
Anything that has mass and occupies space. Anything that has mass and takes up space
Anything that has mass and takes up space Anthing that takes up space and has mass is called?
Anthing that takes up space and has mass is called? Matter is anything that has both
Matter is anything that has both Matter is anything that has mass and
Matter is anything that has mass and Is anything that has mass and takes up space
Is anything that has mass and takes up space Matter is anything that has
Matter is anything that has Whats anything that has mass and takes up space
Whats anything that has mass and takes up space Thể thơ truyền thống
Thể thơ truyền thống Tôn thất thuyết là ai
Tôn thất thuyết là ai Walmart thất bại ở nhật
Walmart thất bại ở nhật






















