Matter Matter Anything that takes up space and






























- Slides: 44


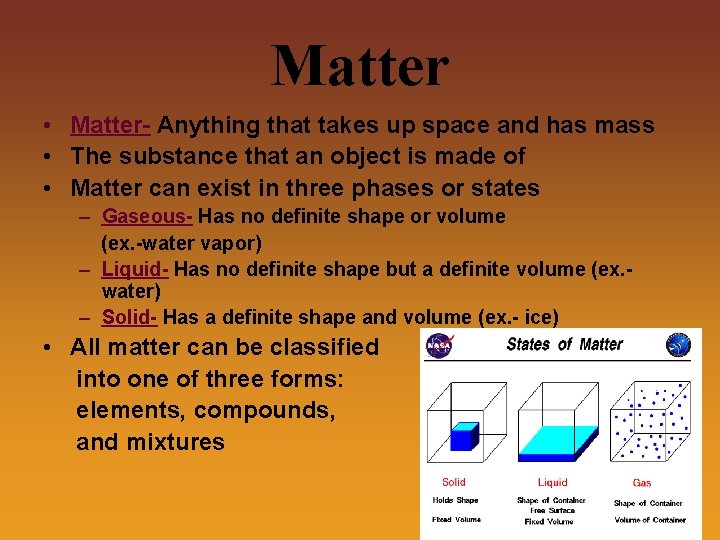
Matter • Matter- Anything that takes up space and has mass • The substance that an object is made of • Matter can exist in three phases or states – Gaseous- Has no definite shape or volume (ex. -water vapor) – Liquid- Has no definite shape but a definite volume (ex. water) – Solid- Has a definite shape and volume (ex. - ice) • All matter can be classified into one of three forms: elements, compounds, and mixtures

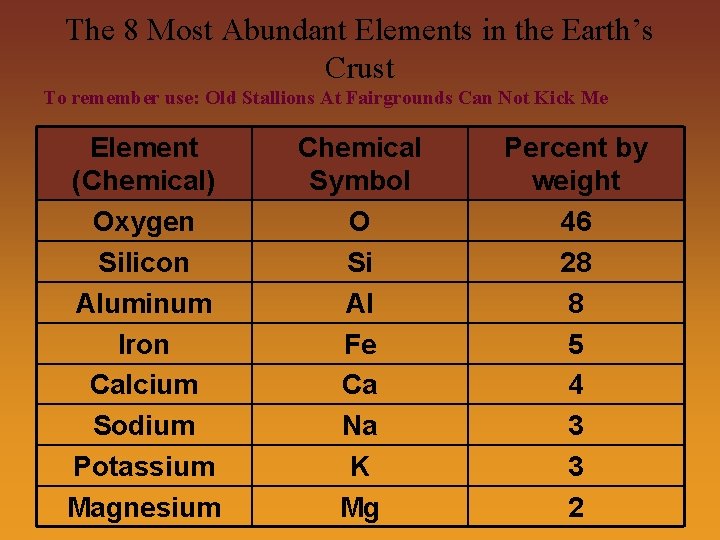
Elements • Element- A substance that cannot be separated into simpler substances by ordinary chemical or physical means • Elements are pure substances, composed of only one type of atom • There are currently 115 known elements (1112, 114, 116, & 118) • 92 elements occur naturally, the rest have been synthetically created in labs • Most of the Earth’s crust is made up of only 8 elements

The 8 Most Abundant Elements in the Earth’s Crust To remember use: Old Stallions At Fairgrounds Can Not Kick Me Element (Chemical) Oxygen Silicon Aluminum Iron Calcium Sodium Potassium Magnesium Chemical Symbol O Si Al Fe Ca Na K Mg Percent by weight 46 28 8 5 4 3 3 2

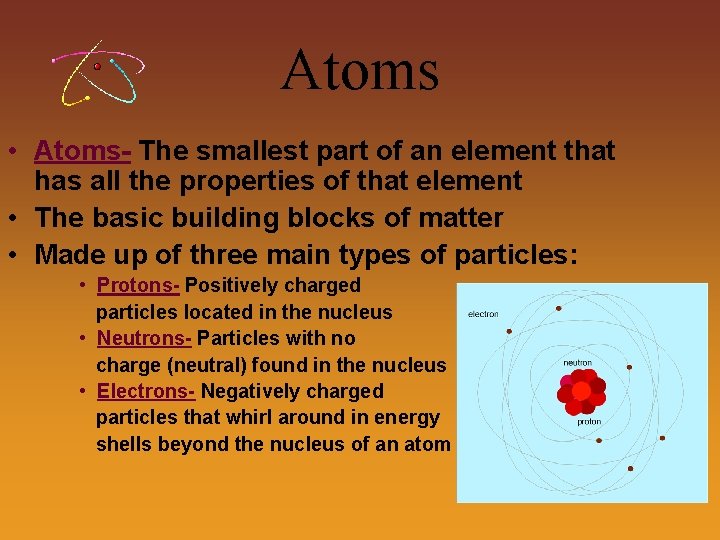
Atoms • Atoms- The smallest part of an element that has all the properties of that element • The basic building blocks of matter • Made up of three main types of particles: • Protons- Positively charged particles located in the nucleus • Neutrons- Particles with no charge (neutral) found in the nucleus • Electrons- Negatively charged particles that whirl around in energy shells beyond the nucleus of an atom

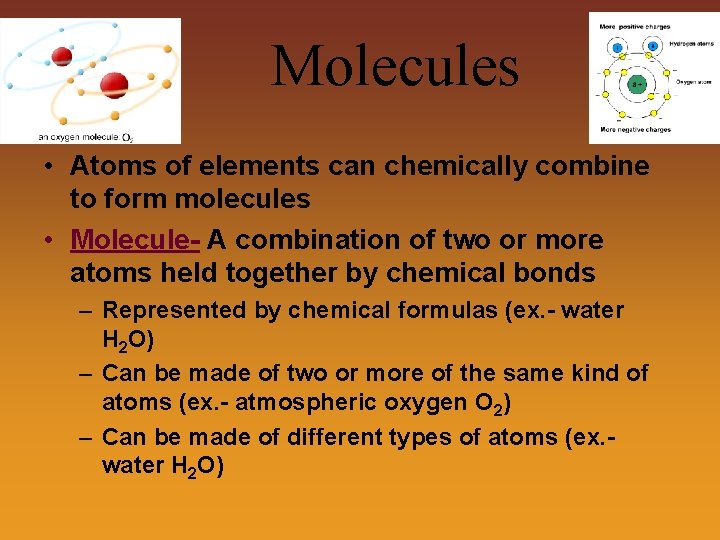
Molecules • Atoms of elements can chemically combine to form molecules • Molecule- A combination of two or more atoms held together by chemical bonds – Represented by chemical formulas (ex. - water H 2 O) – Can be made of two or more of the same kind of atoms (ex. - atmospheric oxygen O 2) – Can be made of different types of atoms (ex. water H 2 O)

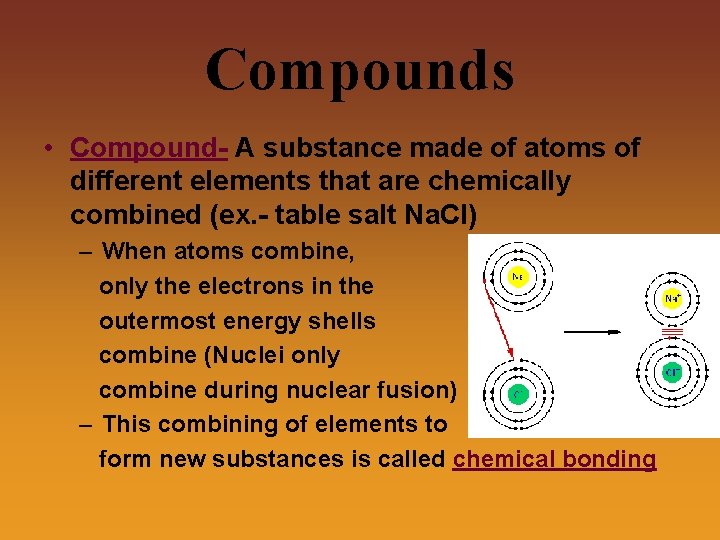
Compounds • Compound- A substance made of atoms of different elements that are chemically combined (ex. - table salt Na. Cl) – When atoms combine, only the electrons in the outermost energy shells combine (Nuclei only combine during nuclear fusion) – This combining of elements to form new substances is called chemical bonding

Mixtures • Mixture- Matter that consists of two or more substances that are physically combined (mixed together) but not chemically combined – Components can be separated out by normal physical means – Example- Dissolving sugar in coffee or trying to combine oil and water

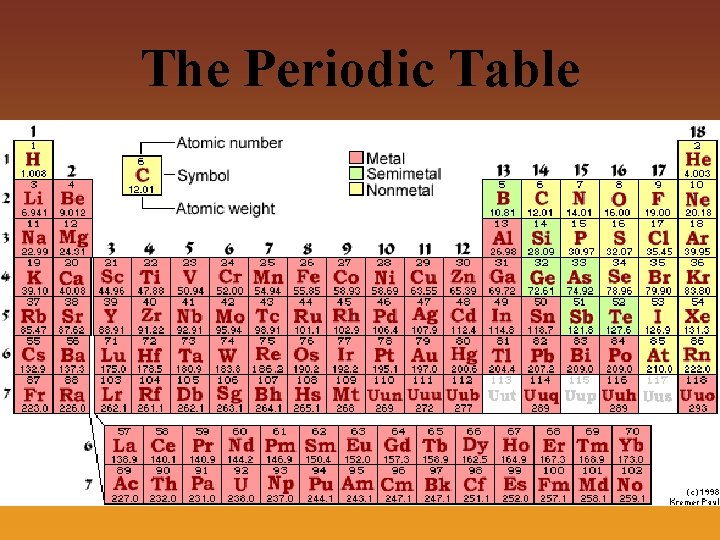
The Periodic Table • Periodic Table- A table in which elements are arranged in order of increasing atomic number – Elements are placed in horizontal rows (called periods) based on atomic number – Elements are placed in vertical columns (called groups) based on having the same number of electrons in their outermost energy shell which gives them similar chemical properties – Periodic Vocabulary: • Atomic number- The number of protons in the nucleus of an atom • Atomic mass- The average mass of an atom for a particular element, based upon the number of protons and neutrons in the nucleus of an atom

The Periodic Table

Minerals • Mineral- A naturally occurring, inorganic solid that has a definite chemical composition and crystal structure • All minerals must: • Occur naturally in/on the Earth • Be inorganic (not formed from living things or the remains of living things) • Be a solid • Have a definite chemical composition • Have a characteristic crystal structure • There about 2500 different kinds of minerals

Formation & Composition of Minerals • Minerals are naturally occurring elements or compounds – Most result from the chemical combination of two or more elements – Ex. - oxygen & silicon combine to form quartz • Native elements- mineral made of only one type of elements – Found uncombined in nature – Ex. - gold, silver, copper, & sulfur • Many minerals form from Magma – Mineral crystals form when magma cools – How and where the magma cools determines the size of the crystals • Mineral crystals may also form from compounds dissolved in a liquid such as water c o p p e r

Identifying Minerals • Minerals have certain physical properties that can be used to identify them • Some properties can be observed just by looking at the mineral • • Color Luster Crystal shape How it breaks • Certain properties can only be determined by testing • • Hardness Streak Specific Gravity Acid test

Mineral Color • The color of a mineral often helps to identify it, but very few minerals can be identified by color alone • Why? – Many minerals have similar colors – Impurities can produce different colors – Some minerals change color due to exposure

Mineral Luster • Luster- Describes the way a mineral reflects light from its surface • All minerals have either a metallic or nonmetallic luster • Metallic luster- When a mineral looks and/or shines like a metal (ex. pyrite) • Nonmetallic lusterwhen a mineral doesn't reflect light or look like a metal (7 types)

Nonmetallic Mineral Lusters 1. Vitreous or Glassy- Quartz 2. Pearly- Mica 3. Resinous or Waxy- Sphalerite 4. Silky- Asbestos 5. Adamantine (hard or unbreakable)- Diamond 6. Greasy or Oily- Talc 7. Dull or Earthy- Sulfur

Crystal Shape • All minerals have a characteristic crystal shape that results from the way atoms or molecules come together when the mineral is forming • The conditions in which the mineral formed, may make it difficult to determine its crystal shape (crystals need room and time to develop) • Crystal- Solid in which the atoms or molecules are arranged in definite repeating pattern

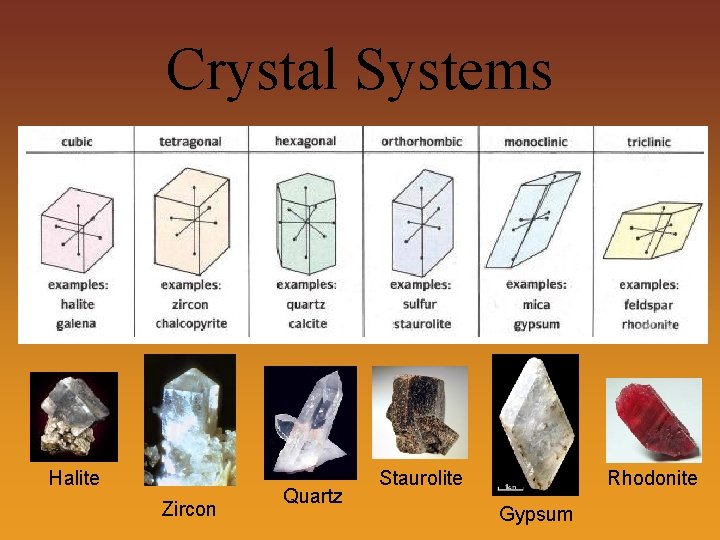
Crystal Systems All mineral have internal atomic patterns in one of 6 possible crystal systems Crystal System Examples Cubic Tetragonal Orthorhombic Fluorite Wulfenite Sulfur Monoclinic Malachite Triclinic Hexagonal Halite Zircon Topaz Orthoclase feldspar Amazonite Plagioclase feldspar Quartz Tourmaline

Crystal Systems Halite Zircon Quartz Staurolite Rhodonite Gypsum

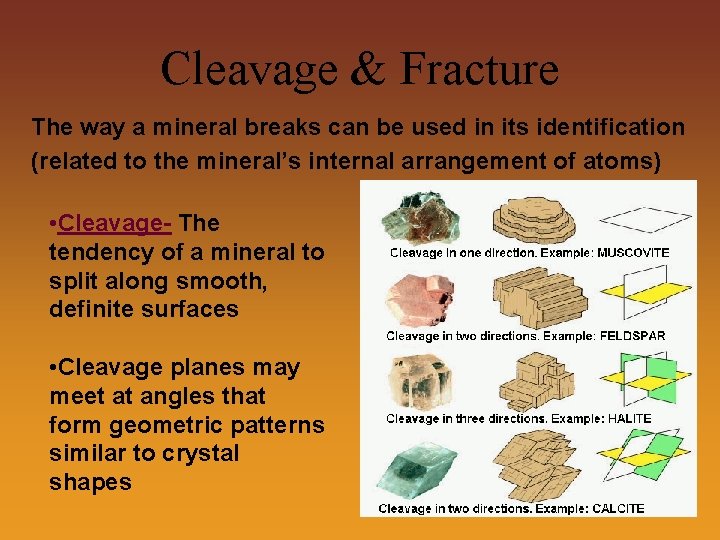
Cleavage & Fracture The way a mineral breaks can be used in its identification (related to the mineral’s internal arrangement of atoms) • Cleavage- The tendency of a mineral to split along smooth, definite surfaces • Cleavage planes may meet at angles that form geometric patterns similar to crystal shapes

Cleavage & Fracture • Fracture- When a mineral breaks along a rough or jagged surface • Types of fracture: 1. Conchoidal or shell-like- Smooth curved surface, like the inside of a clam shell (ex. - Obsidian) 2. Fibrous or splintery- Jagged surface with sharp uneven edges (ex. - Copper) 3. Uneven or irregular- Surface is generally rough all over (ex. - serpentine)

Mineral Hardness • Hardness- The ability of a mineral to resist being scratched • Mohs’ scale- A reference guide for determining the hardness of minerals that uses ten minerals arranged in order of increasing hardness The Girls Came From All Over Quebec To Collect Diamonds

Determining the Hardness of a Mineral To determine the hardness of an unknown mineral: 1. 2. 3. 4. 5. Rub the mineral against the surface of a mineral (or other substance) that you know the hardness of If the unknown mineral scratches the known mineral, it is harder than the known mineral If the unknown mineral is scratched by the other mineral, then it is softer than that mineral If neither mineral scratches the other, they have the same hardness Based upon the scratch test, minerals are assigned a number between 1 and 10 (with. 5 increments)

Streak • Streak- The color of a mineral in its powdered form – Produced when a mineral is rubbed against a hard, rough surface – Usually a piece of unglazed porcelain know as a streak plate • Even though the color of a mineral may vary, its streak does not • For many minerals, its color and the color of its streak may be different

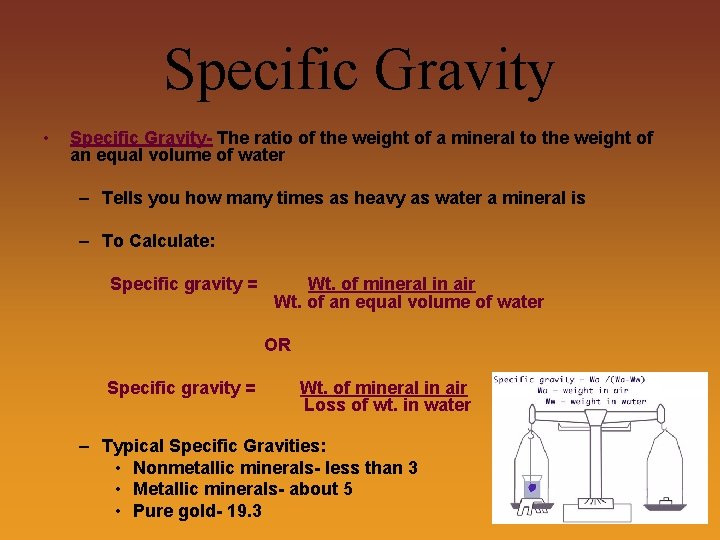
Specific Gravity • Specific Gravity- The ratio of the weight of a mineral to the weight of an equal volume of water – Tells you how many times as heavy as water a mineral is – To Calculate: Specific gravity = Wt. of mineral in air Wt. of an equal volume of water OR Specific gravity = Wt. of mineral in air Loss of wt. in water – Typical Specific Gravities: • Nonmetallic minerals- less than 3 • Metallic minerals- about 5 • Pure gold- 19. 3

The Acid Test • Some minerals fizz or bubble when hydrochloric acid (HCl) is dropped on them – The HCl reacts with the mineral and produces carbon dioxide (CO 2) gas – Can be used to identify calcite, copper, and other carbonate minerals

Special Properties of Minerals 1. Magnetism- Magnetite 2. Taste- Halite (Rock Salt) 3. Fluorescence (Glows under UV light)- Scheelite and some Calcites 4. Phosphorescence (Glows without UV light)- Sphalerite 5. Radioactivity- Uranium 6. Double refraction (Splits light rays into two parts, causing double vision)- Calcite 7. Smell- Sulfur

From Minerals to Rocks • “Minerals are to rocks as letters are to words” (Quote from Professor R. Lingner, WSC) • Rocks- Hard substances composed of one or more minerals • Usually made of more than one type of mineral • May contain naturally occurring substances that would not be considered a mineral • Are grouped according to how they form as either igneous, sedimentary, or metamorphic

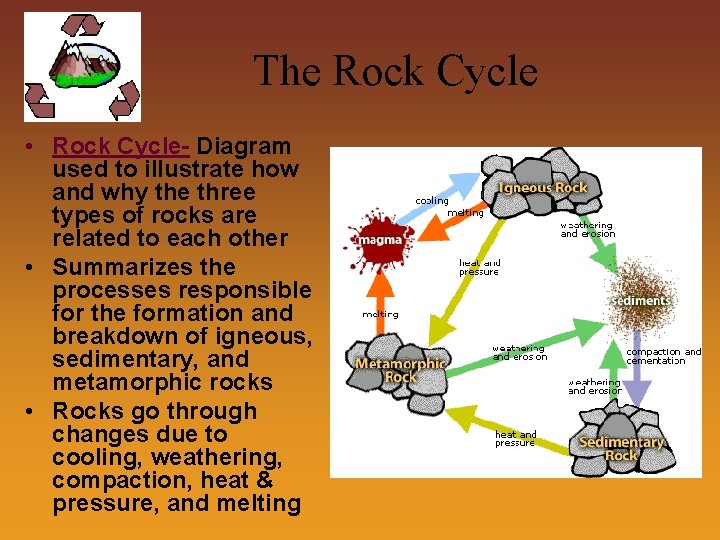
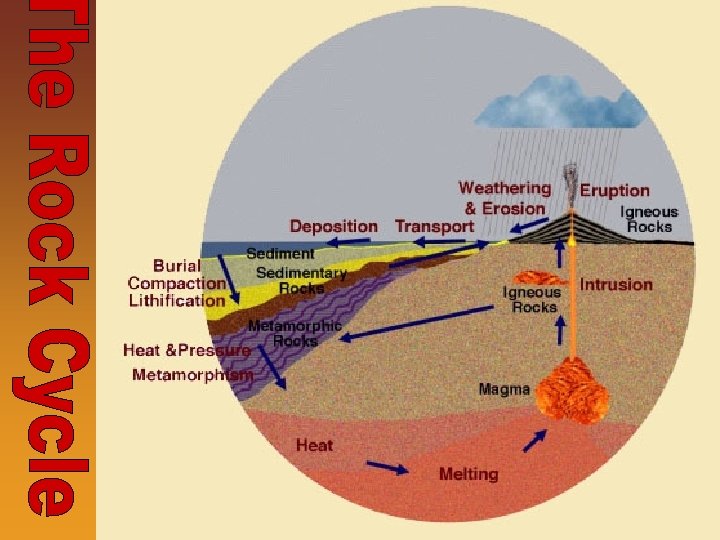
The Rock Cycle • Rock Cycle- Diagram used to illustrate how and why the three types of rocks are related to each other • Summarizes the processes responsible for the formation and breakdown of igneous, sedimentary, and metamorphic rocks • Rocks go through changes due to cooling, weathering, compaction, heat & pressure, and melting


Igneous Rocks • Igneous rocks- Rocks formed by the cooling of molten lava or magma • Classified according to their composition and texture – Composition refers to the type of minerals that are present – Texture refers to the shape, size and distribution of the minerals in the rock

Why Igneous Rocks Have Different Textures • How and where the lava or magma cools determines the size of the mineral crystals • The longer it takes to cool, the larger the crystals • Classification based on where they form: – Extrusive rocks- Formed from lava that cools quickly on the earth’s surface • Produces fine-grained or glassy textured rocks • Ex. – Basalt or Obsidian – Intrusive rocks- Formed from magma that cools slowly below the Earth’s surface • Produces coarse-grained rocks • Ex. - Granite and Pegmatite

Basic Textures of Igneous Rocks • Glassy- Forms on the surface from lava which solidifies too rapidly for crystals to develop • Shiny and look like glass • Extrusive • Ex. - Obsidian • Fine-grained- Forms on the surface from lava that cools and solidifies rapidly • Crystals are usually too small to see without a magnification • Extrusive • Ex. - Basalt

Basic Textures of Igneous Rocks • Coarse-grained- Forms deep within the crust from magma which cools and solidifies slowly • Consist of large crystals of roughly the same size • Intrusive • Ex. - Granite • Porphyry- Formation involves two stages of cooling, one at depth and the other at the surface • Have two different textures, large crystals are scattered on a background of smaller crystals • Ex. - Andesite porphyry

Sedimentary Rocks • • Sedimentary rocks- Rocks formed from the compacting and cementing of sediments Placed into one of three categories based upon the origin of their sediments 1. Clastic rocks- fragments of other rocks 2. Organic rocks- remains of organisms 3. Chemical rocks- minerals precipitated from solutions

Clastic Sedimentary Rocks • Formed from fragments of previously existing rocks • Sediments form as rock undergo weathering and erosion • Before new rocks form, the sediments are transported, compacted, and finally cemented together • Further classifies based on the size of the sediments • Conglomerate- Rounded pebbles • Sandstone- small, sand-sized sediments • Shale- particles of mud and clay

Organic Sedimentary Rocks • Formed directly or indirectly from material that was once living • Two of the most important organic sedimentary rocks are limestone and coal – Organic limestone- Form from the shells of creatures like clams and certain microorganism – Coal- Formed from plants that were buried in swamps millions of years ago (plants →peat →coal)

Chemical Sedimentary Rocks Formed when a water body dries up, leaving behind minerals that were previously dissolved in the water Common Chemical Sedimentary Rocks Name of Rock Limestone Rock Salt Rock Gypsum Formation Formed from tiny grains of calcite deposited in water bodies Formed when water evaporated from a salt lake or ocean bay which was cut off by sandbars

Features of Sedimentary Rocks • Stratification- When rock beds are arranged in layers – New layers form when the type of sediments change – Rock layers are separated by bedding planes • Fossils- The remains of organisms preserved in rocks

Features of Sedimentary Rocks • Ripple marks- Formed by the action of winds or moving water on sand deposits • Mud cracks- Develop when deposits of wet clay dry and contract • Concretions- Solid ball or disk-like features that form when silica precipitates from solution • Geodes- Partially hollow, ball-like objects which have crystals (usually quartz) growing inward from a hard silica rim

Metamorphic Rocks • Rocks changed in form as a result of chemical reactions, heat and/or pressure – May form from igneous, sedimentary, or other metamorphic rocks – During metamorphism, a rock’s texture, mineral composition and chemical composition may be changed

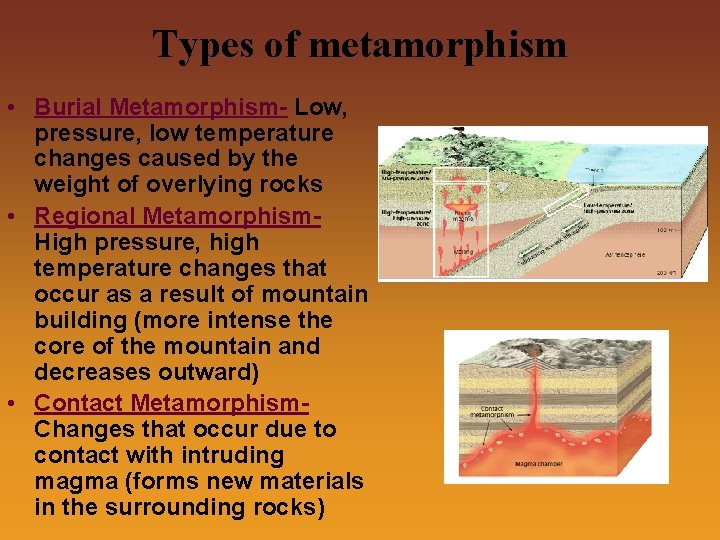
Types of metamorphism • Burial Metamorphism- Low, pressure, low temperature changes caused by the weight of overlying rocks • Regional Metamorphism. High pressure, high temperature changes that occur as a result of mountain building (more intense the core of the mountain and decreases outward) • Contact Metamorphism. Changes that occur due to contact with intruding magma (forms new materials in the surrounding rocks)

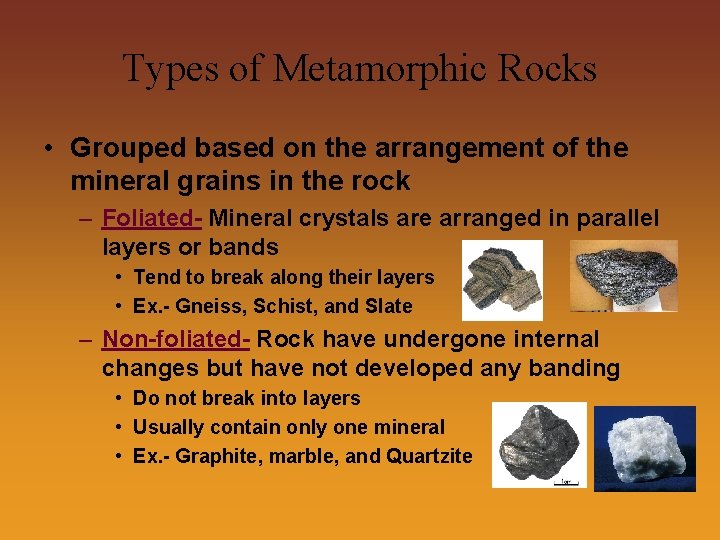
Types of Metamorphic Rocks • Grouped based on the arrangement of the mineral grains in the rock – Foliated- Mineral crystals are arranged in parallel layers or bands • Tend to break along their layers • Ex. - Gneiss, Schist, and Slate – Non-foliated- Rock have undergone internal changes but have not developed any banding • Do not break into layers • Usually contain only one mineral • Ex. - Graphite, marble, and Quartzite
