MATTER What is matter Matter is anything that






















- Slides: 37

MATTER ~ What is matter? ~ Matter is anything that has ______ __ MASS & TAKES UP _____ SPACE ~ Name & draw 5 things that ARE matter.

Is AIR matter? Is LIGHT matter? Does air have MASS? Does light have MASS? Does air TAKE UP SPACE? Does light TAKE UP SPACE? Complete the chart in your notes, and draw and label pictures for each item.

Atoms, Elements, Compounds, and Ions • Atom • basic building block of all matter • Element • Substance that consists of only one type of atom. • Molecule has two different atoms. • Compound • substance that consists of more than one type of element. • Ion • substance that has a positive or negative charge


4 Principles of the Particle Theory matter is made of tiny particles. All particles of one substance are the same. - Different substances are made of different particles. The particles are always moving. - The more energy the particles have, the faster they move. There attractive forces between particles. - These forces are stronger when the particles are closer together.

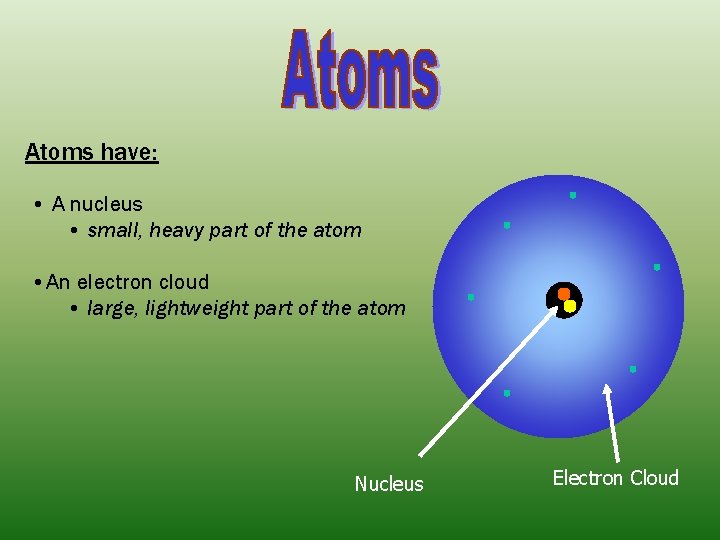
Atoms have: • A nucleus • small, heavy part of the atom • An electron cloud • large, lightweight part of the atom Nucleus Electron Cloud

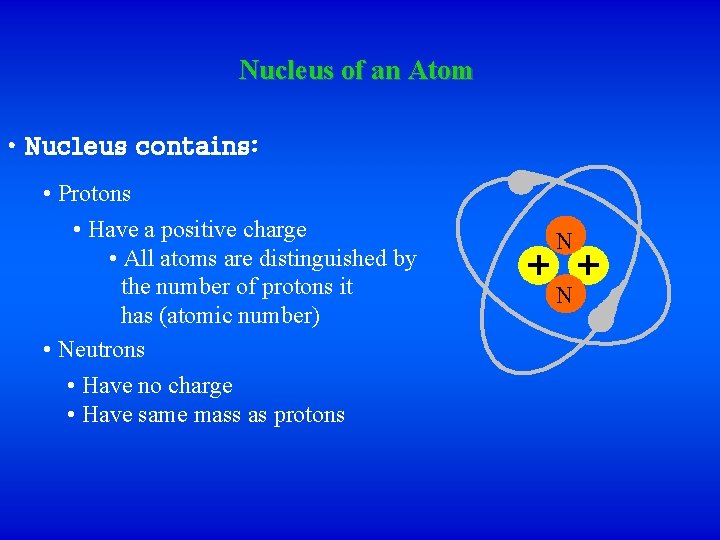
Nucleus of an Atom • Nucleus contains: • Protons • Have a positive charge • All atoms are distinguished by the number of protons it has (atomic number) • Neutrons • Have no charge • Have same mass as protons N N N

Electron Cloud of an Atom An electron cloud contains: • Electrons e- • have a negative charge e- • are contained within shells of the electron cloud • orbit the nucleus of the atom • have a very small mass compared to protons and neutrons • determine bonding properties of substance

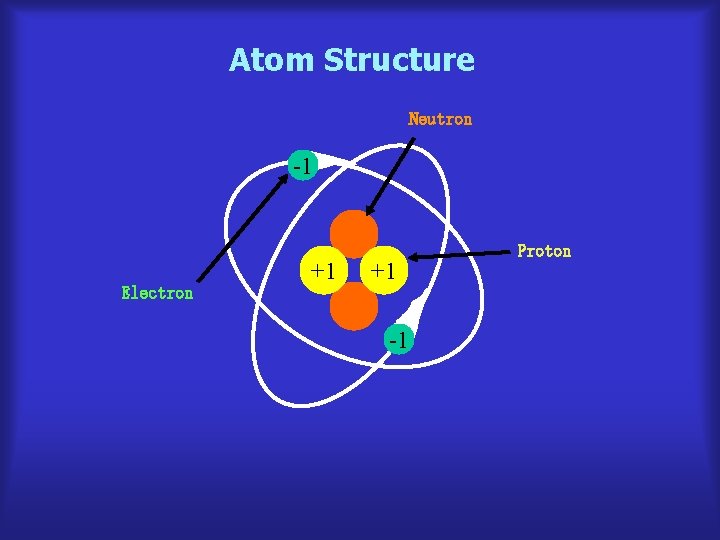
Atom Structure Neutron -1 Electron +1 +1 -1 Proton

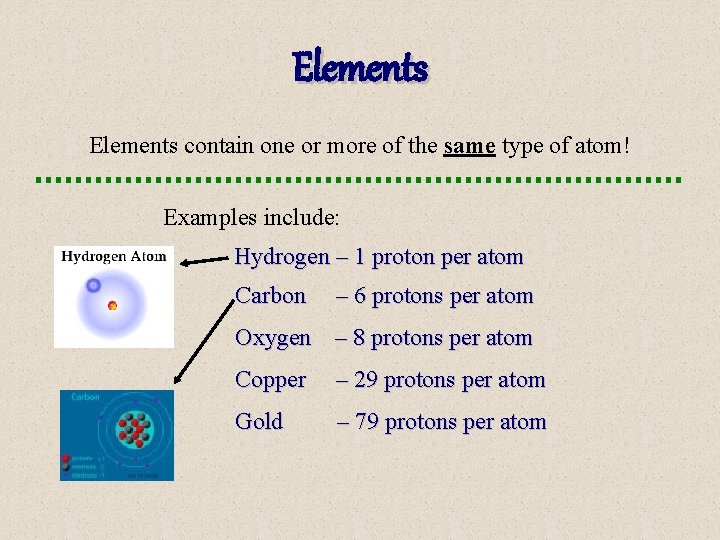
Elements contain one or more of the same type of atom! Examples include: Hydrogen – 1 proton per atom Carbon – 6 protons per atom Oxygen – 8 protons per atom Copper – 29 protons per atom Gold – 79 protons per atom

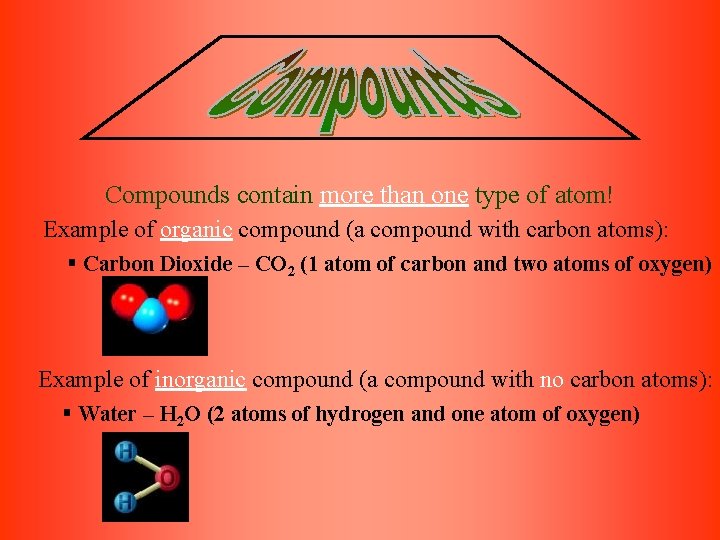
Compounds contain more than one type of atom! Example of organic compound (a compound with carbon atoms): § Carbon Dioxide – CO 2 (1 atom of carbon and two atoms of oxygen) Example of inorganic compound (a compound with no carbon atoms): § Water – H 2 O (2 atoms of hydrogen and one atom of oxygen)

- + An ion is an atom or group of atoms with a positive or negative charge!! § A particle with a neutral charge has the same number of protons and electrons. § An ion does not have the same number of electrons and protons. § Examples of ions: • He+ - A helium atom that is missing one electron. The atom has one more proton than electron, and must have a positive charge. • CO 32 - - Carbonate has two more electrons than protons

Lets take a look deep inside

Lets take a look deep inside

Lets take a look deep inside

Lets take a look deep inside





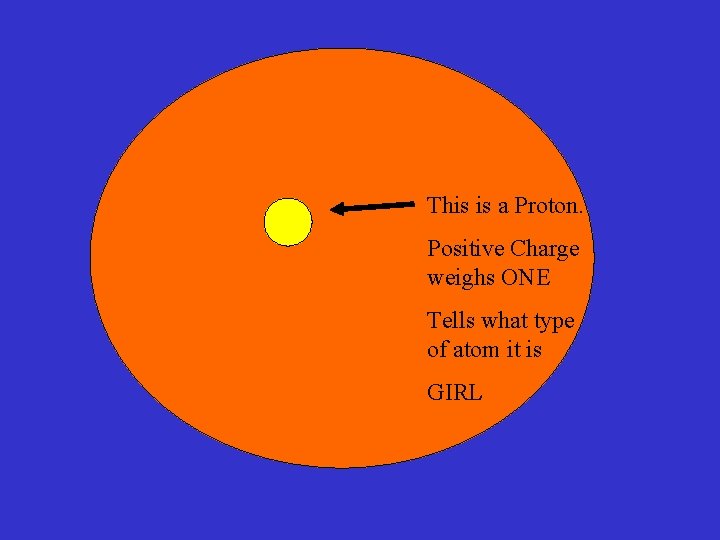
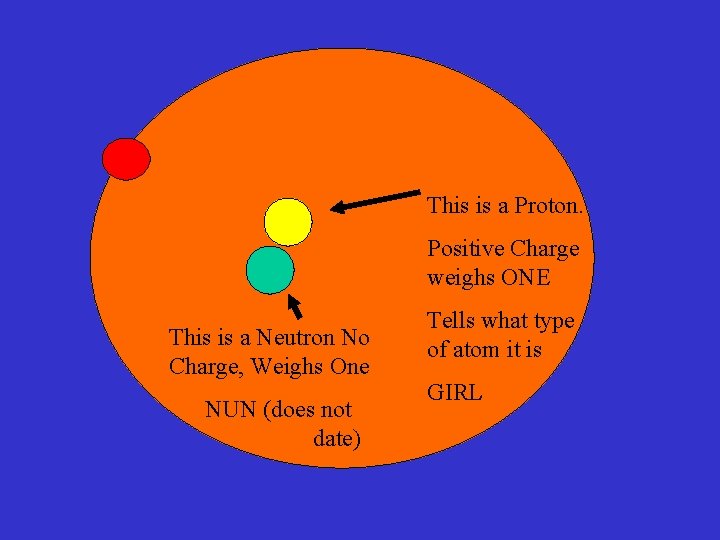
This is a Proton. Positive Charge weighs ONE Tells what type of atom it is GIRL

This is a Proton. Positive Charge weighs ONE Tells what type of atom it is GIRL

This is a Proton. Positive Charge weighs ONE Tells what type of atom it is GIRL

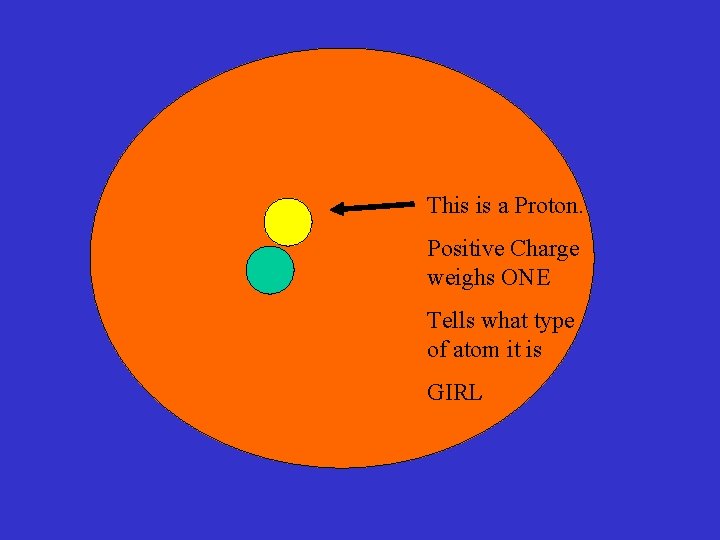
This is a Proton. Positive Charge weighs ONE This is a Neutron No Charge, Weighs One NUN (does not date) Tells what type of atom it is GIRL

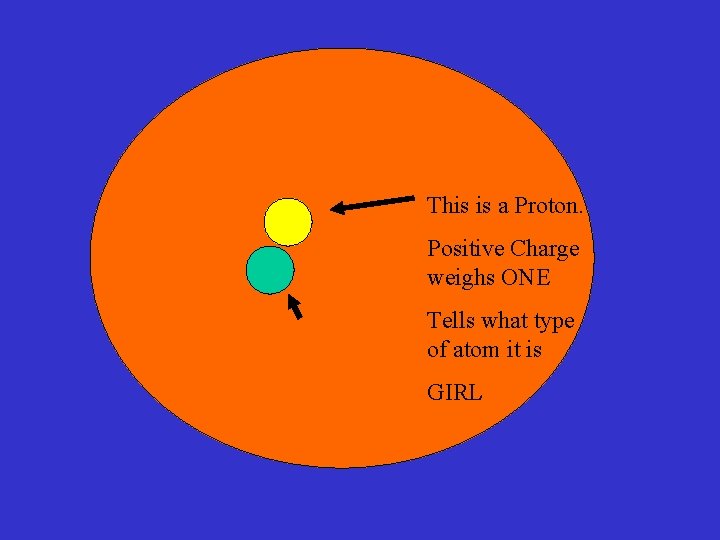
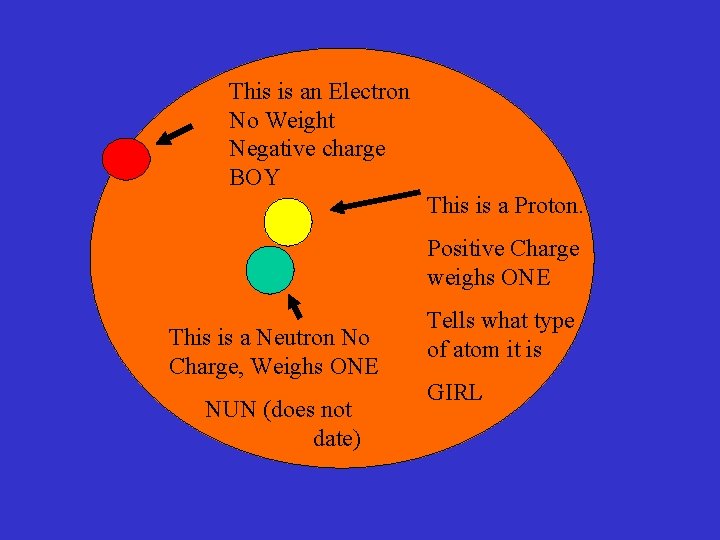
This is an Electron No Weight Negative charge BOY This is a Proton. Positive Charge weighs ONE This is a Neutron No Charge, Weighs ONE NUN (does not date) Tells what type of atom it is GIRL


Element Guide

Element Guide 2

Element Guide 2

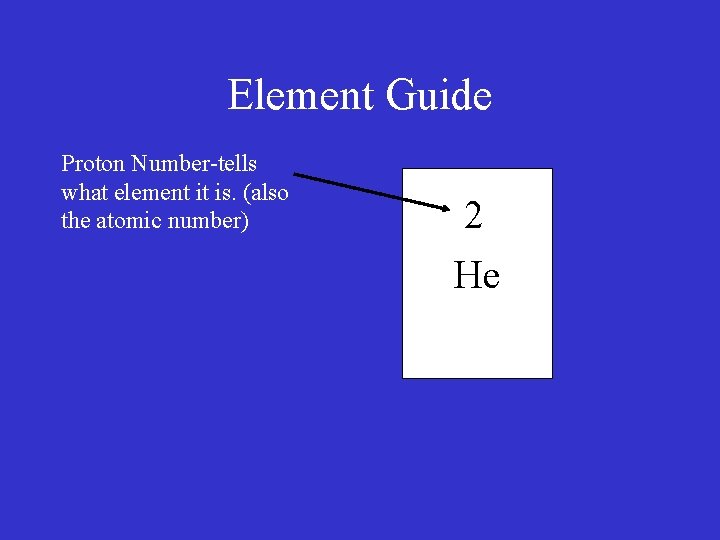
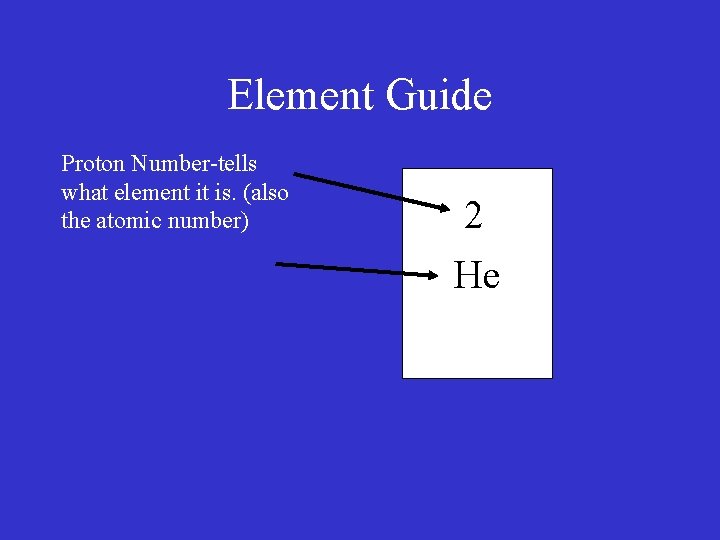
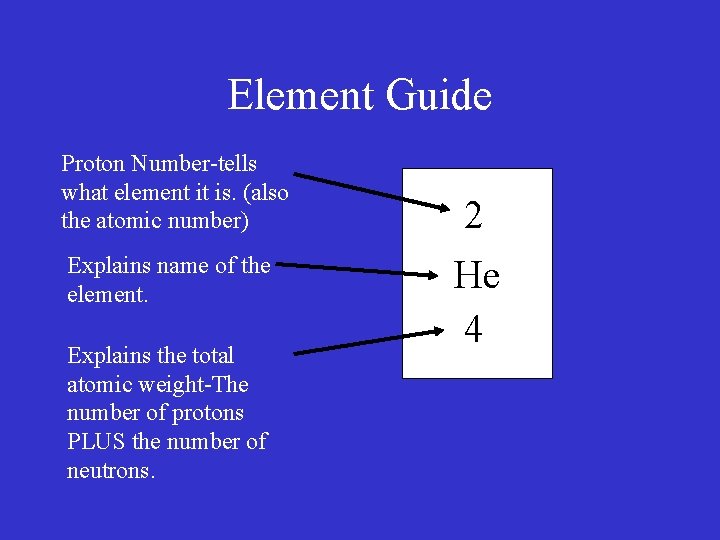
Element Guide Proton Number-tells what element it is. (also the atomic number) 2

Element Guide Proton Number-tells what element it is. (also the atomic number) 2 He

Element Guide Proton Number-tells what element it is. (also the atomic number) 2 He

Element Guide Proton Number-tells what element it is. (also the atomic number) Explains name of the element. 2 He

Element Guide Proton Number-tells what element it is. (also the atomic number) Explains name of the element. 2 He 4

Element Guide Proton Number-tells what element it is. (also the atomic number) Explains name of the element. 2 He 4

Element Guide Proton Number-tells what element it is. (also the atomic number) Explains name of the element. Explains the total atomic weight-The number of protons PLUS the number of neutrons. 2 He 4

Element Guide Proton Number-tells what element it is. (also the atomic number) Explains name of the element. Explains the total atomic weight-The number of protons PLUS the number of neutrons. 2 He 4
 Tôn thất thuyết là ai
Tôn thất thuyết là ai Phân độ lown
Phân độ lown Walmart thất bại ở nhật
Walmart thất bại ở nhật Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block nhĩ thất độ 2 mobitz 2
Block nhĩ thất độ 2 mobitz 2 Tìm vết của đường thẳng
Tìm vết của đường thẳng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Matter is defined as anything that
Matter is defined as anything that Ductility defintion
Ductility defintion Matter anything that
Matter anything that Whats anything that has mass and takes up space
Whats anything that has mass and takes up space Matter is anything that has
Matter is anything that has No matter anything
No matter anything Anything that has mass
Anything that has mass Is anything that has mass and takes up space
Is anything that has mass and takes up space Matter anything that
Matter anything that Matter is anything that
Matter is anything that Anything that has matter and takes up space
Anything that has matter and takes up space Matter is anything that
Matter is anything that Matter anything that
Matter anything that Is anything that has mass and takes up space.
Is anything that has mass and takes up space. Matter anything that
Matter anything that Matter is anything that:
Matter is anything that: No matter anything
No matter anything No matter anything
No matter anything Matter anything that
Matter anything that Matter is anything that has mass and
Matter is anything that has mass and Is oil more dense than water
Is oil more dense than water Anything that has mass and take up space
Anything that has mass and take up space Matter is anything that
Matter is anything that What is anything that occupies space and has mass?
What is anything that occupies space and has mass? Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key