Chapter 8 Periodic Properties of the Elements Chapter

















































- Slides: 86

Chapter 8 Periodic Properties of the Elements Chapter 8

Chapter 8 The Quantum-Mechanical Model of the Atom 8. 1 8. 2 8. 3 8. 4 8. 5 8. 6 8. 7 Nerve Signal Transmission The Development of the Periodic Table Electron Configurations: How Electrons Occupy Orbitals Electron Configurations, Valence Electrons, and the Periodic Table The Explanatory Power of the Quantum-Mechanical Model Periodic Trends in the Size of Atoms and Effective Nuclear Charge Ions: Electron Configurations, Magnetic Properties, Ionic Radii, and Ionization Energy 8. 8 Electron Affinities and Metallic Character 8. 9 Some Examples of Periodic Chemical Behavior: The Alkali Metals, the Halogens, and the Noble Gases 2

Section 8. 1 Nerve Signal Transmission Periodic Properties • Nerve cells – Pumps and channels can differentiate Na+ and K+ ions – Maintain a gradient of these ions • Pump sodium out • Pump potassium in – How can the pump tell the difference? 3

Section 8. 1 Nerve Signal Transmission Periodic Properties • Nerve cells – How can the pump tell the difference? • Potassium ion has larger radius • Pumps are sensitive to this difference 4

Section 8. 1 Nerve Signal Transmission Periodic Properties • The relative size of Na and K ions is an example of a periodic property: • is predictable based on an element’s position in the periodic table. • In this chapter, we examine several periodic properties – atomic radius, – ionization energy, – and electron affinity. 5

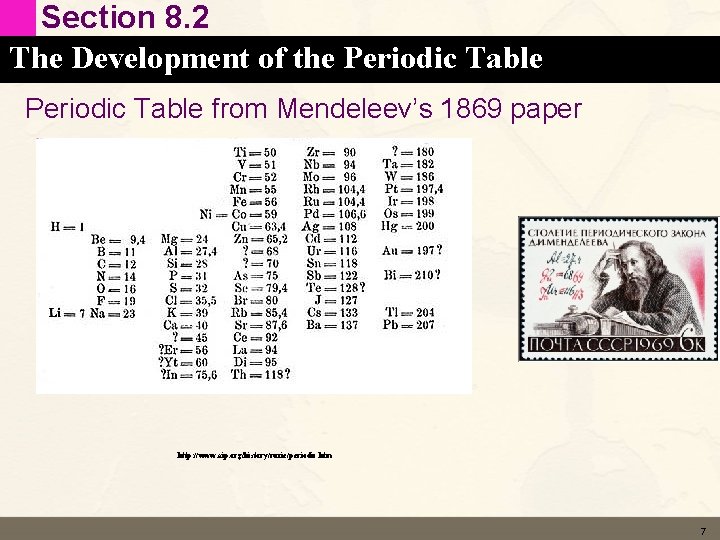
Section 8. 2 The Development of the Periodic Table Macroscopic vs Subatomic World Elements were discovered and purified one at a time Late 1800 s scientists started to group chemicals together that all behaved the same way 1872, Dimitri Mendeleev arranged the 60 know elements into groups with similar properties and arranged them in order of increasing atomic mass 6

Section 8. 2 The Development of the Periodic Table from Mendeleev’s 1869 paper http: //www. aip. org/history/curie/periodic. htm 7

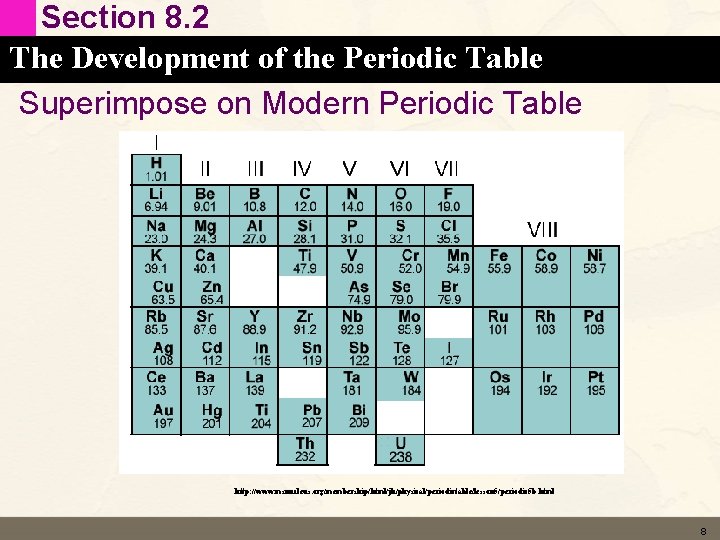
Section 8. 2 The Development of the Periodic Table Superimpose on Modern Periodic Table http: //www. msnucleus. org/membership/html/jh/physical/periodictable/lesson 5/periodic 5 b. html 8

Section 8. 2 The Development of the Periodic Table and the Scientific Approach • Observations led to the organization of the table and the periodic law • The table (as a picture or expression of the periodic law) had predictive and descriptive power. Describes what happens. • But it did not explain why • Quantum mechanical theory explains why 9

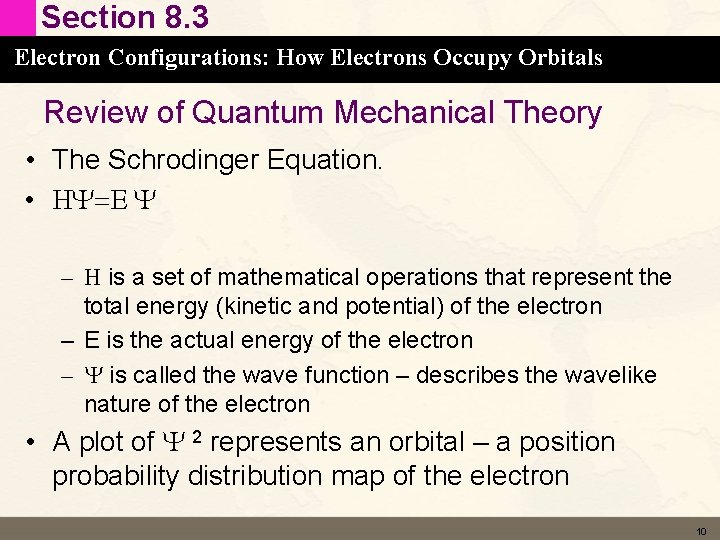
Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Review of Quantum Mechanical Theory • The Schrodinger Equation. • HY=E Y – H is a set of mathematical operations that represent the total energy (kinetic and potential) of the electron – E is the actual energy of the electron – Y is called the wave function – describes the wavelike nature of the electron • A plot of Y 2 represents an orbital – a position probability distribution map of the electron 10

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Review of Quantum Mechanical Theory • Orbitals are represented by three dimensional plots of the wave functions • Each orbital is described by three quantum numbers • Principle quantum number (n) – size/energy of the orbital • Angular momentum quantum number (l) – shape of orbital • Magnetic quantum number – (ml) – orientation of orbital 11

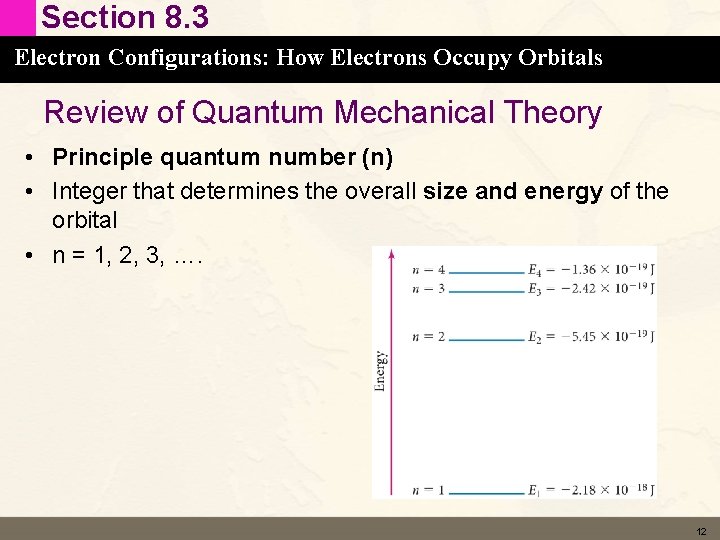
Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Review of Quantum Mechanical Theory • Principle quantum number (n) • Integer that determines the overall size and energy of the orbital • n = 1, 2, 3, …. 12

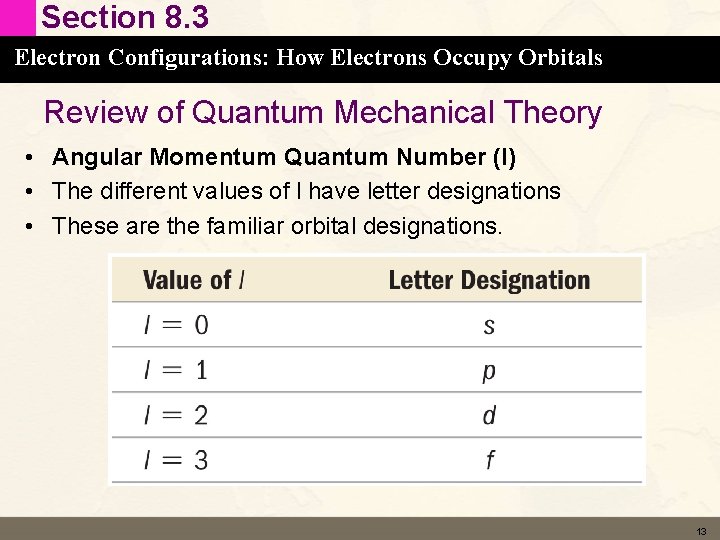
Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Review of Quantum Mechanical Theory • Angular Momentum Quantum Number (l) • The different values of l have letter designations • These are the familiar orbital designations. 13

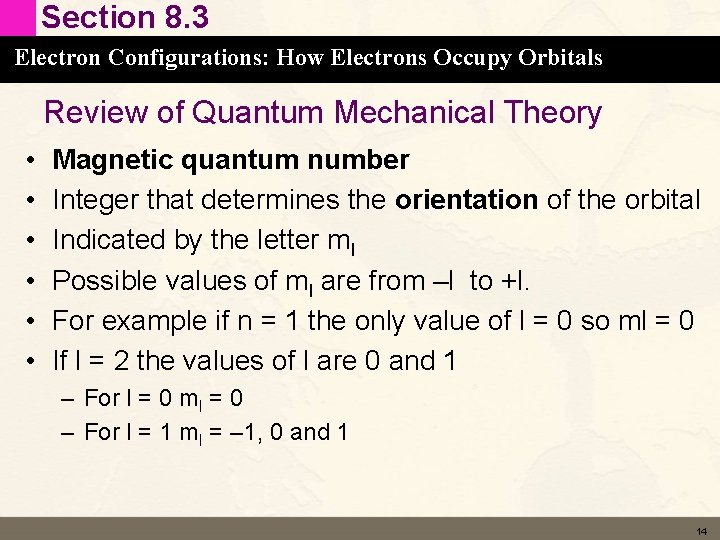
Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Review of Quantum Mechanical Theory • • • Magnetic quantum number Integer that determines the orientation of the orbital Indicated by the letter ml Possible values of ml are from –l to +l. For example if n = 1 the only value of l = 0 so ml = 0 If l = 2 the values of l are 0 and 1 – For l = 0 ml = 0 – For l = 1 ml = – 1, 0 and 1 14

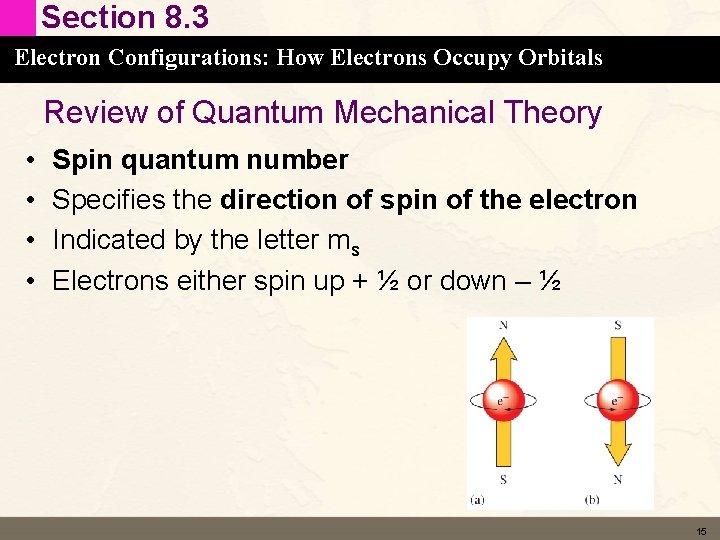
Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Review of Quantum Mechanical Theory • • Spin quantum number Specifies the direction of spin of the electron Indicated by the letter ms Electrons either spin up + ½ or down – ½ 15

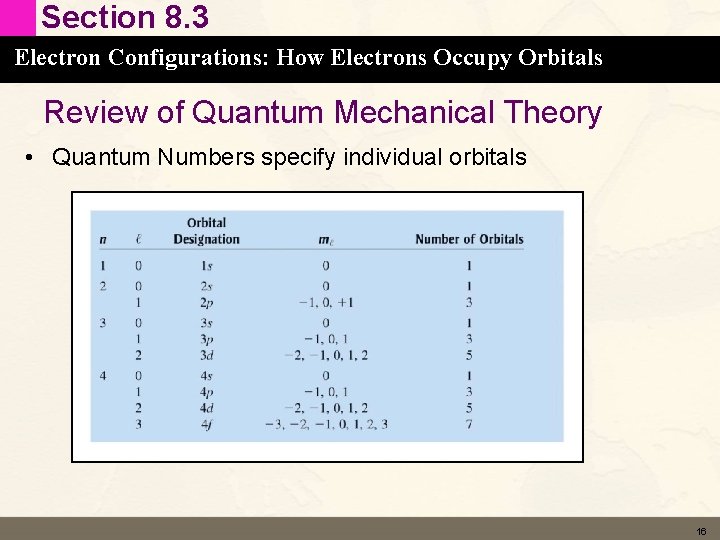
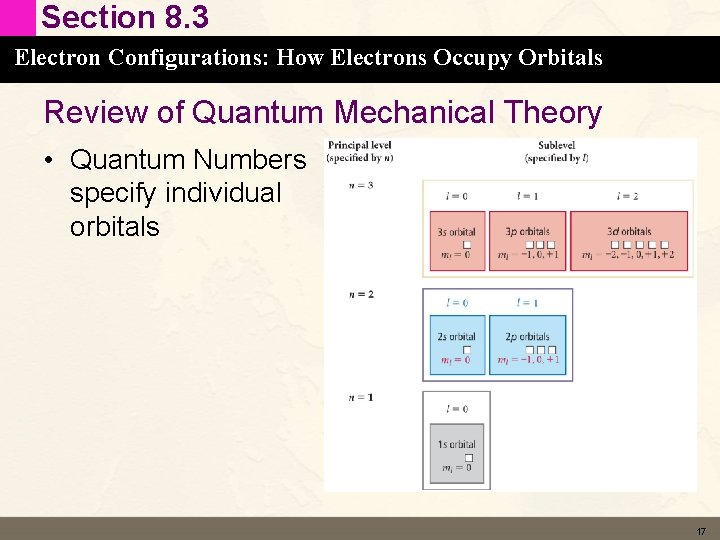
Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Review of Quantum Mechanical Theory • Quantum Numbers specify individual orbitals 16

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Review of Quantum Mechanical Theory • Quantum Numbers specify individual orbitals 17

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Concept Check For principal quantum level n = 3, determine the number of allowed subshells (different values of l), and give the designation (number and letter) of each. 18

Section 7. 5 Electron Configurations: How Electrons Occupy Orbitals Concept Check For l = 2, determine the magnetic quantum numbers (ml) and the number of orbitals. 19

Section 7. 5 Electron Configurations: How Electrons Occupy Orbitals Concept Check Each set of quantum numbers below is supposed to specify an orbital. However each set contains one quantum number that is not allowed. Replace the quantum number that is not allowed with one that is allowed A. n = 3, l = 3, ml = +2 B. n= 2, l = 1, ml = – 2 C. n = 1, l = 1, ml = 0 20

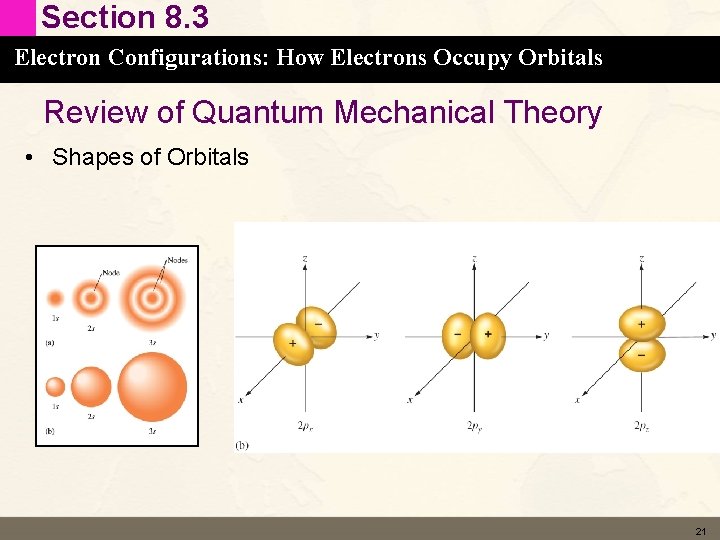
Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Review of Quantum Mechanical Theory • Shapes of Orbitals 21

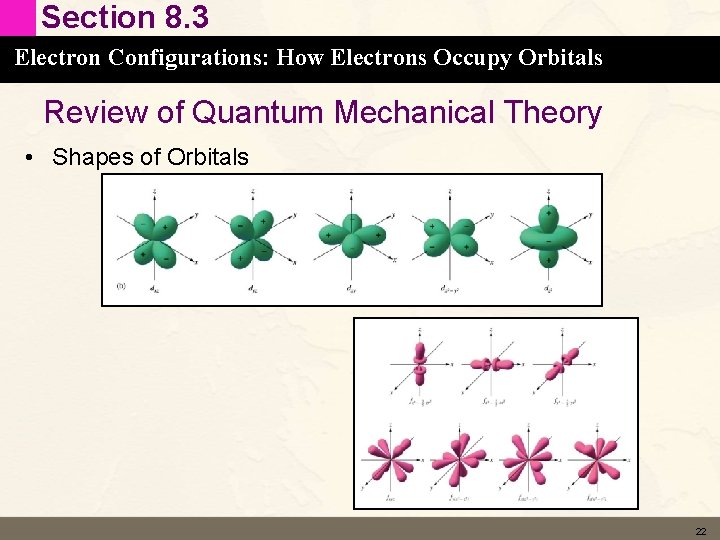
Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Review of Quantum Mechanical Theory • Shapes of Orbitals 22

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Electron Configurations • Remember the periodic law was the what • QM theory is the why • The way electrons occupy the orbitals explains – The observations that led to the arrangement of the PT – Periodicity – Chemical behavior 23

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Electron Configurations • Electrons occupy orbitals. • An electron configuration shows which orbitals an electron occupies for a particular atom. • This electron configuration is for the ground state (lowest energy state) for a hydrogen atom. • H 1 s 1 24

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Orbital Diagrams • Another way to depict the electron configuration is with an orbital diagram which symbolizes the electron as an arrow and the orbital as a box • H 1 s • The direction of the arrow represents the electrons spin (which is quantized). There are only two possibilities – up or down. • The spin is represented by the forth quantum number – ms = +1/2 and – 1/2 (forth quantum number) 25

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Multielectron Atoms • The solutions to the Schroedinger Equation were specifically for the hydrogen atom – which has a single electron • Solutions to the Schroedinger Equation for multielectron atoms produce orbitals that are “hydrogen-like”. • In order to see how electrons occupy these hydrogen-like orbitals we have to take into account electron spin and the effect of sublevel splitting. 26

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Electron Spin and the Pauli Exclusion Principle • How do we depict the Helium atom which has two electrons He 1 s 2 He 1 s • The spins of the two electrons align according to the Pauli Exclusion Principle • No two electrons in an atom can have the same four quantum numbers 27

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Electron Spin and the Pauli Exclusion Principle • How does the Pauli Principle account for the way electrons align in this orbital diagram He 1 s For these two electrons the first three quantum numbers would be the same n = 1, l = 0 and ml = 0 In order for them too not have the same set of four the last one has too be different 28

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Electron Spin and the Pauli Exclusion Principle He 1 s Electron 1 Electron 2 29

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Sublevel Splitting in Multielectron Atoms • In the H atom the Energy of the orbital depends only on the value of n • The 3 s, 3 p and 3 d orbitals all have the same energy • These energy levels are degenerate 30

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Sublevel Splitting in Multielectron Atoms • In a multielectron atom the energies of these three levels are NOT degenerate • Their energy depends primarily on the value of l • the energies of the sublevels are split • For a given value of n • E(s orbital) < E(p orbital) < E(d orbital) < E(f orbital) 31

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Sublevel Splitting in Multielectron Atoms • To understand energy level splitting lets look at three things that affect an electron in the vicinity of a nucleus • 1. Coulomb’s Law • 2. Shielding • 3. Pentration 32

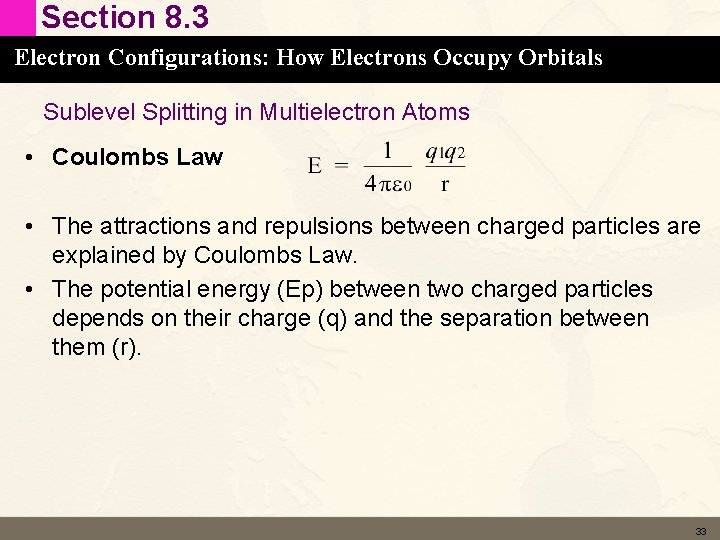
Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Sublevel Splitting in Multielectron Atoms • Coulombs Law • The attractions and repulsions between charged particles are explained by Coulombs Law. • The potential energy (Ep) between two charged particles depends on their charge (q) and the separation between them (r). 33

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Sublevel Splitting in Multielectron Atoms • Coulombs Law • For like charges (two electrons) Ep is positive (unstable) when they are close together and decreases as they are separated. – Therefore like charges repel each other • For opposite charges (protons and electrons) Ep is negative and (stable) becomes more negative (even more stable) as they become closer together. – Therefore opposite charges attract each other 34

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Sublevel Splitting in Multielectron Atoms • Shielding • In multielectron atoms the electron feels both the attraction of the nucleus and the repulsion of the other electrons. • Repulsion of one electron by other electrons = shielding or screening that electron from the full effects of the nuclear charge. 35

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Sublevel Splitting in Multielectron Atoms • Shielding • Li+ ion (3 P and 2 e–) • If we bring a third electron towards this nucleus it does not feel the 3+ charge • It feels 1+ (3+ from the nucleus and 2– from the electrons) • Effective nuclear charge Zeff = +1 36

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Sublevel Splitting in Multielectron Atoms • Penetration • As the electron comes closer it penetrates the electron cloud of the 1 s electrons and begins to feel the full 3+ charge of the nucleus because it is less shielded. • As an outer electron undergoes penetration into a region occupied by inner electrons it feels and greater Zeff and has lower Energy according to Coulombs Law. 37

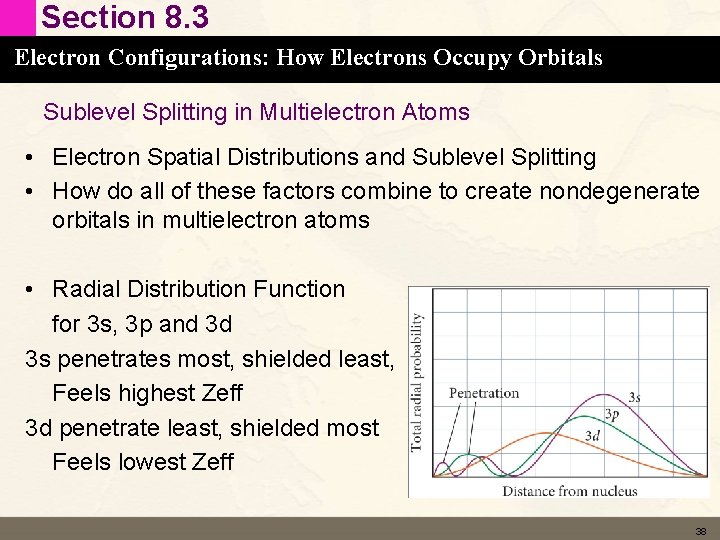
Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Sublevel Splitting in Multielectron Atoms • Electron Spatial Distributions and Sublevel Splitting • How do all of these factors combine to create nondegenerate orbitals in multielectron atoms • Radial Distribution Function for 3 s, 3 p and 3 d 3 s penetrates most, shielded least, Feels highest Zeff 3 d penetrate least, shielded most Feels lowest Zeff 38

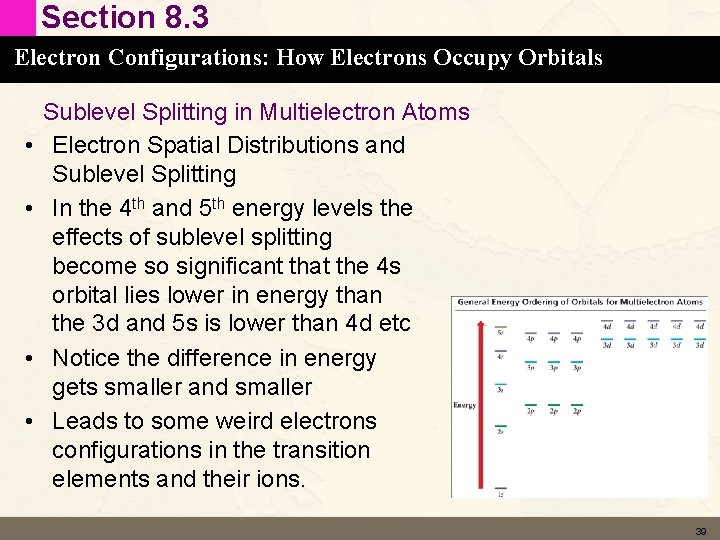
Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals • • Sublevel Splitting in Multielectron Atoms Electron Spatial Distributions and Sublevel Splitting In the 4 th and 5 th energy levels the effects of sublevel splitting become so significant that the 4 s orbital lies lower in energy than the 3 d and 5 s is lower than 4 d etc Notice the difference in energy gets smaller and smaller Leads to some weird electrons configurations in the transition elements and their ions. 39

Section 8. 3 Electron Configurations: How Electrons Occupy Orbitals Conceptual Connection Which Statement is true A. An orbital that penetrates into the region occupied by core electrons is more shielded from nuclear charge than an orbital that does not penetrate and will therefore have a higher energy. B. An orbital that penetrates into the region occupied by core electron is less shielded from nuclear charge than an orbital that does not penetrate and will therefore have a higher energy. C. An orbital that penetrates into the region occupied by core electron is less shielded from nuclear charge than an orbital that does not penetrate and will therefore have a lower energy. D. An orbital that penetrates into the region occupied by core electrons is more shielded from nuclear charge than an orbital that does not penetrate and will therefore have a lower energy. 40

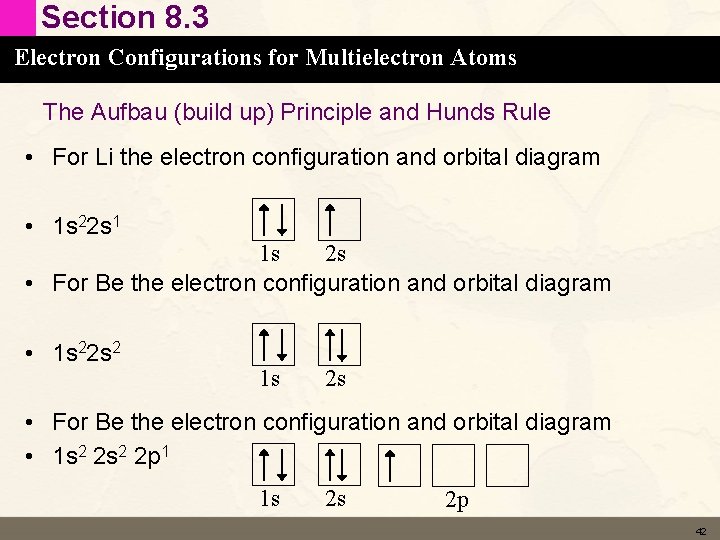
Section 8. 3 Electron Configurations for Multielectron Atoms The Aufbau (build up) Principle and Hunds Rule • Now that we know the energy of ordering of orbitals we can determine the ground state electron configuration for the rest of the elements. • We know the electrons occupy the lowest energy orbitals available to them and only 2 electrons (of opposite spin) are allowed per orbital. 41

Section 8. 3 Electron Configurations for Multielectron Atoms The Aufbau (build up) Principle and Hunds Rule • For Li the electron configuration and orbital diagram • 1 s 22 s 1 1 s 2 s • For Be the electron configuration and orbital diagram • 1 s 22 s 2 1 s 2 s • For Be the electron configuration and orbital diagram • 1 s 2 2 p 1 1 s 2 s 2 p 42

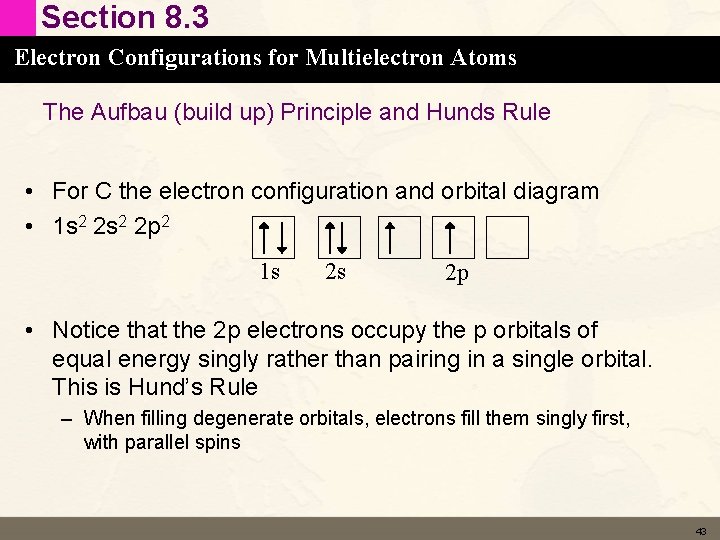
Section 8. 3 Electron Configurations for Multielectron Atoms The Aufbau (build up) Principle and Hunds Rule • For C the electron configuration and orbital diagram • 1 s 2 2 p 2 1 s 2 s 2 p • Notice that the 2 p electrons occupy the p orbitals of equal energy singly rather than pairing in a single orbital. This is Hund’s Rule – When filling degenerate orbitals, electrons fill them singly first, with parallel spins 43

Section 8. 3 Electron Configurations for Multielectron Atoms Learning check • Write the electron configuration and orbital diagram for N, O, F and Ne 44

Section 8. 3 Electron Configurations for Multielectron Atoms Think – pair - share • At first glance the electron configuration and the orbital diagram seem to give the same information. • What is different about them and why do you think it might be important? 45

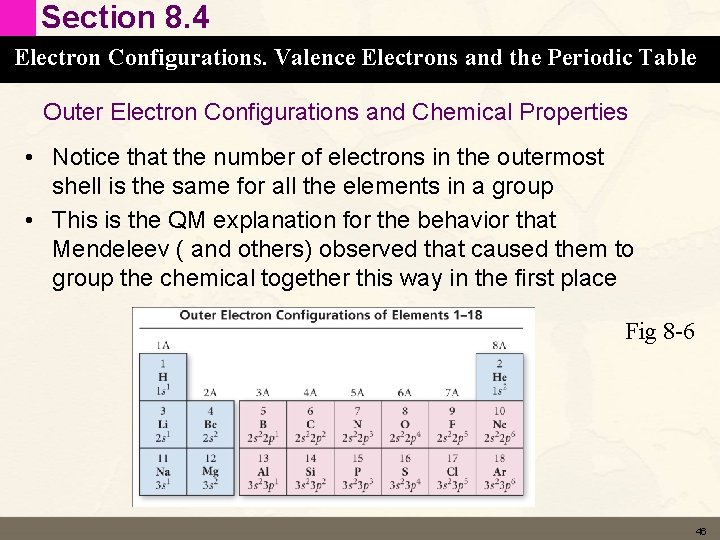
Section 8. 4 Electron Configurations. Valence Electrons and the Periodic Table Outer Electron Configurations and Chemical Properties • Notice that the number of electrons in the outermost shell is the same for all the elements in a group • This is the QM explanation for the behavior that Mendeleev ( and others) observed that caused them to group the chemical together this way in the first place Fig 8 -6 46

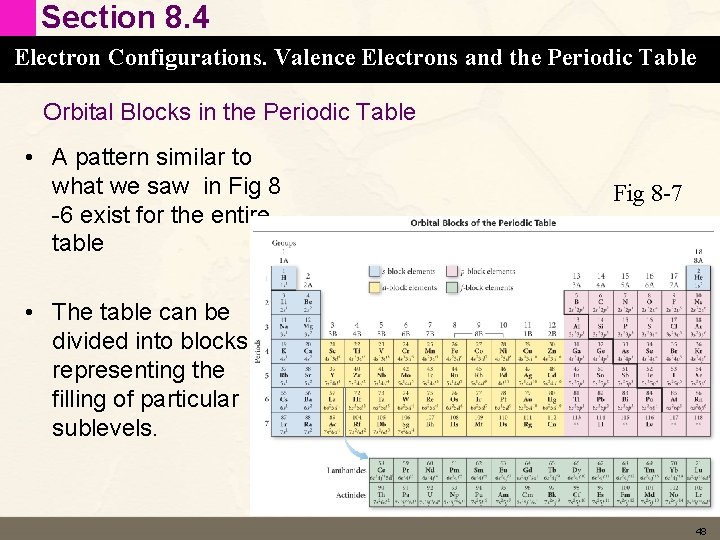
Section 8. 4 Electron Configurations. Valence Electrons and the Periodic Table Valence vs Core electrons • Valence electrons are those that are important in bonding – For main group elements they are the same electrons that we saw in the last slide that are in the outermost shell – For transition elements we also include the outermost d electrons in the valence electrons • Core electrons are all the rest of the electrons in the completed principle energy levels and completed d and f sublevels below the valence electrons 47

Section 8. 4 Electron Configurations. Valence Electrons and the Periodic Table Orbital Blocks in the Periodic Table • A pattern similar to what we saw in Fig 8 -6 exist for the entire table Fig 8 -7 • The table can be divided into blocks representing the filling of particular sublevels. 48

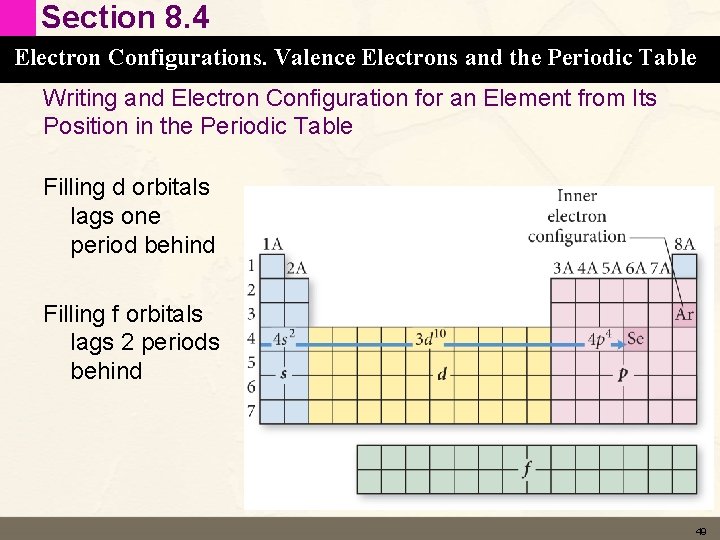
Section 8. 4 Electron Configurations. Valence Electrons and the Periodic Table Writing and Electron Configuration for an Element from Its Position in the Periodic Table Filling d orbitals lags one period behind Filling f orbitals lags 2 periods behind 49

Section 8. 4 Electron Configurations. Valence Electrons and the Periodic Table Writing and Electron Configuration for an Element from Its Position in the Periodic Table • Valence electrons are those that are important in 50

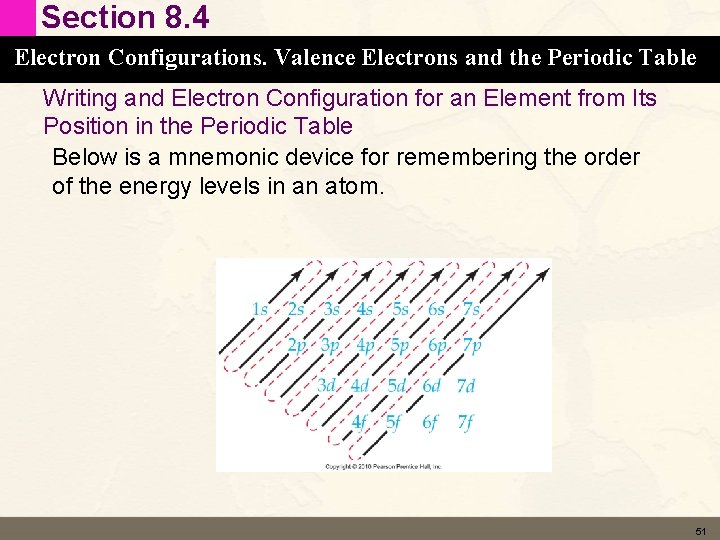
Section 8. 4 Electron Configurations. Valence Electrons and the Periodic Table Writing and Electron Configuration for an Element from Its Position in the Periodic Table Below is a mnemonic device for remembering the order of the energy levels in an atom. 51

Section 8. 4 Electron Configurations. Valence Electrons and the Periodic Table Writing and Electron Configuration for an Element from Its Position in the Periodic Table So what does this look like Write the expected electron configurations for Ba 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 2 3 d fills in the 4 th period 4 d fills in the 5 th period 52

Section 8. 4 Electron Configurations. Valence Electrons and the Periodic Table Learning check Write the expected electron configurations for Eu 53

Section 8. 4 Electron Configurations. Valence Electrons and the Periodic Table Noble Gas Electron configurations • For a really long electron configuration like this • Eu 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 24 f 7 • It is convenient to use what is called and abbreviated or noble gas electron configuration. • The core electrons are represented by the symbol for the previous noble gas in the periodic table (Xe) • And only the core electrons are written out • Eu = [Xe] 6 s 24 f 7 54

Section 8. 4 Electron Configurations. Valence Electrons and the Periodic Table Writing and Electron Configuration for an Element from Its Position in the Periodic Table • Summarizing Orbital Filling 1. Number of electrons = number of protons (atomic number) 2. Electrons occupy orbitals so as to minimize the energy of the atom – lower energy orbitals first. 3. Orbitals can only hold 2 electrons each (opposite spin) (Pauli exclusion principle) 4. When orbitals of equal energy are available electrons occupy these orbitals singly with parallel spins then start to form pairs (Hund’s rule) 55

Section 8. 5 The Explanatory Power of the Quantum-Mechanical Model Periodicity • The chemical properties of elements are largely determined by the number of valence electrons they contain. • The properties of chemicals are periodic because the number of valence electrons is periodic. 56

Section 8. 5 The Explanatory Power of the Quantum-Mechanical Model Stability of Noble gases • Not the number 8 that is magical – it is the stability that is associated with that number – when a quantum level is full the overall energy of the electrons occupying that level is low. • No more stability by reacting – so they don’t • Elements near the noble gases – group 1 and 7 – highly reactive – only have to gain or lose a single electron to attain the highly stable noble gas configuration 57

Section 8. 6 Periodic Trends in the Size of Atoms and Effective Nuclear Charge Atomic Radius • Since quantum mechanical orbitals are really just probabilities we have to determine atomic radius in the lab. • non bonding atomic radius (van der Waals radius) determine distance between atomic nucleii from density – can be done in a frozen sample • Covalent or bonding atomic radius – Nonmetals – ½ the distance between two of the atoms bonded together – Metals – ½ the distance between two atoms in a crystal of the metal 58

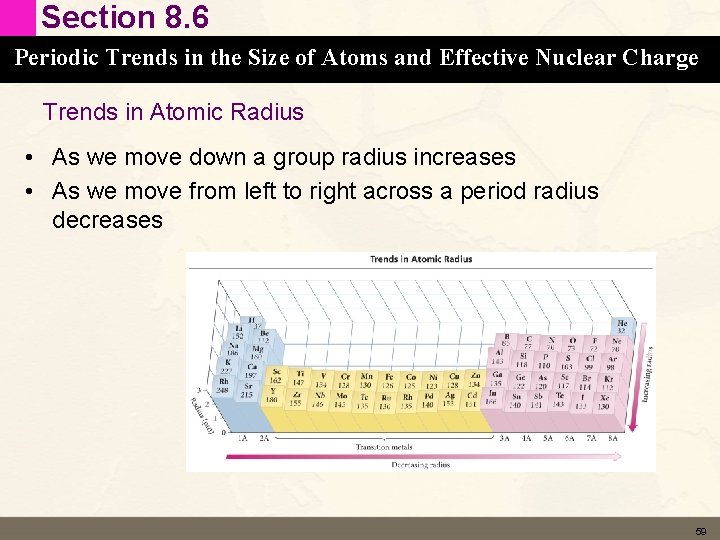
Section 8. 6 Periodic Trends in the Size of Atoms and Effective Nuclear Charge Trends in Atomic Radius • As we move down a group radius increases • As we move from left to right across a period radius decreases 59

Section 8. 6 Periodic Trends in the Size of Atoms and Effective Nuclear Charge Trends in Atomic Radius • As we move down a group radius increases – Radius largely determined by the highest principle quantum number of the valence electrons • As we move from left to right across a period radius decreases – This trend is more subtle and involves the effects of effective nuclear charge and shielding 60

Section 8. 6 Periodic Trends in the Size of Atoms and Effective Nuclear Charge Moving Down a Group Radius Increases • Atomic radius largely determined by the valence electrons – As we move down a column in the periodic table the highest principle quantum number (n) of the valence electrons increases – Valence electrons occupy larger orbitals which results in larger atoms 61

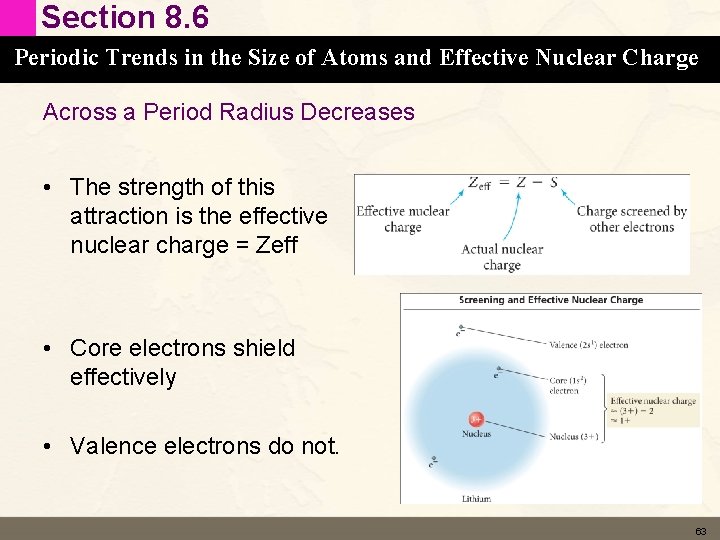
Section 8. 6 Periodic Trends in the Size of Atoms and Effective Nuclear Charge Across a Period Radius Decreases • Effective Nuclear Charge – As we move left to right we add electrons to the valence shell of the same principle energy level. – We also add an equal number of protons to the nucleus. – The radius of the atom is determined by strength of the attraction of the protons in the nucleus for the valence electrons. 62

Section 8. 6 Periodic Trends in the Size of Atoms and Effective Nuclear Charge Across a Period Radius Decreases • The strength of this attraction is the effective nuclear charge = Zeff • Core electrons shield effectively • Valence electrons do not. 63

Section 8. 6 Periodic Trends in the Size of Atoms and Effective Nuclear Charge Across a Period Radius Decreases • As we move (for example) across period 3 the number of protons increases from 11 to 17 – These protons are attracting 11 – 17 electrons. – Seems like the size should be the same • But only the 10 core electrons shield effectively so the single Na valence electron only feels a Zeff of +1 where each Mg valence electron feels a Zeff of +2 • Mg has a smaller radius than Na • Etc across the period 64

Section 8. 6 Periodic Trends in the Size of Atoms and Effective Nuclear Charge Atomic Radii and the Transition Elements • As we move across a period in the transition elements the radius stays fairly constant • The number of electrons in the outermost principle energy level stays fairly constant • 4 s fills before 3 d for example • Even though electrons get added they go into the nhighest – 1 orbital 65

Section 8. 3 Electron Configurations for Multielectron Atoms Concept check • Choose the larger atom in each pair • A. Sn or I • B. Cr or W • C. F or Se 66

Section 8. 7 Ions: Electron Configurations, Magnetic Properties, Ionic Radii and Ionization Energy Electron Configurations and Magnetic Properties of Ions • For main group elements predicting the electron configuration of ions is straightforward. • For cations we subtract the number of electrons equal to the magnitude of the ion • last electron added = first electron lost • Na = 1 s 22 p 63 s 1 Na+ = 1 s 22 p 6 • For anions we add the number of electrons equal to the magnitude of the ion • Cl – = 1 s 22 p 63 s 23 p 5 67

Section 8. 7 Ions: Electron Configurations, Magnetic Properties, Ionic Radii and Ionization Energy Electron Configurations and Magnetic Properties of Ions • For transition metal cations the trend is different. • Even though the d orbitals of the n – 1 energy level fill after the ns orbitals – 3 d fill after 4 s • When ions are formed ns electrons are lost before n – 1 d – 4 s are lost before 3 d 68

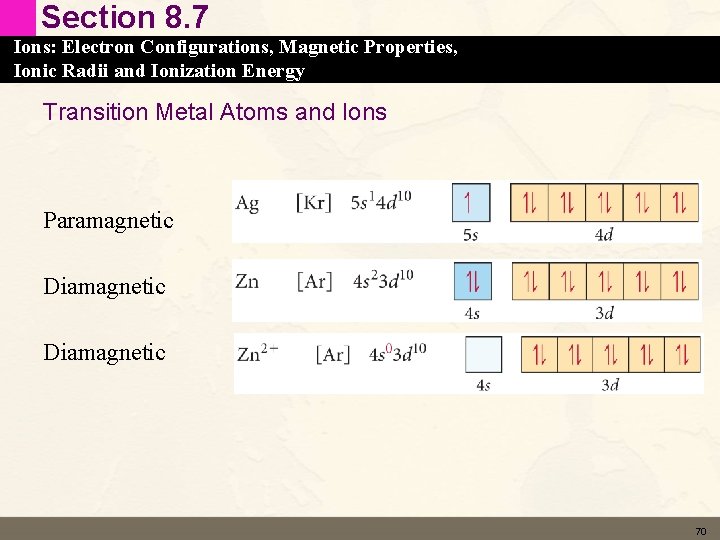
Section 8. 7 Ions: Electron Configurations, Magnetic Properties, Ionic Radii and Ionization Energy Paramagnetic and Diamagnetic • An electron spins on its axis and creates a little tiny magnet • Any unpaired electron in an electron configuration will make a metal paramagnetic – Paramagnetic – attracted to a magnetic field • Paired electrons make a metal diamagnetic – Diamagnetic – repelled by a magnetic field 69

Section 8. 7 Ions: Electron Configurations, Magnetic Properties, Ionic Radii and Ionization Energy Transition Metal Atoms and Ions Paramagnetic Diamagnetic 70

Section 8. 3 Electron Configurations for Multielectron Atoms Concept Check • Write the orbital diagram for Ni 2+ and determine if the ion is paramagnetic or diamagnetic 71

Section 8. 7 Ions: Electron Configurations, Magnetic Properties, Ionic Radii and Ionization Energy Ionic Radii • Cations are smaller than the corresponding atom • Anions are larger than the corresponding atom 72

Section 8. 7 Ions: Electron Configurations, Magnetic Properties, Ionic Radii and Ionization Energy • Ionization energy is the amount of energy required to remove an electron from an atom or ion in the gaseous state. • Na (g) Na+ (g) + 1 e – IE 1 = 496 k. J/mole • Na+ (g) Na 2+ (g) + 1 e – IE 2 = 4560 k. J/mole – Why the gaseous state? 73

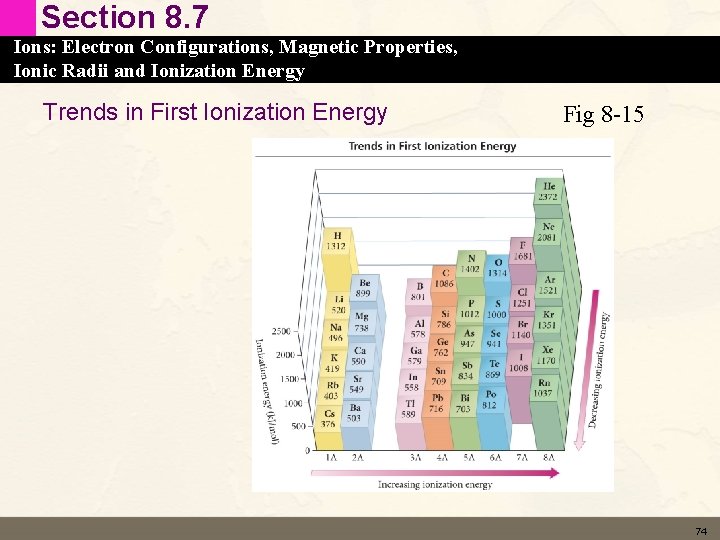
Section 8. 7 Ions: Electron Configurations, Magnetic Properties, Ionic Radii and Ionization Energy Trends in First Ionization Energy Fig 8 -15 74

Section 8. 7 Ions: Electron Configurations, Magnetic Properties, Ionic Radii and Ionization Energy Trends in First Ionization Energy • Ionization energy generally: • Decreases down a column – Valence electrons held less tightly • Increases across a row – Valence electrons experience higher Zeff 75

Section 8. 7 Ions: Electron Configurations, Magnetic Properties, Ionic Radii and Ionization Energy Exceptions to Trends in First Ionization Energy Think – Pair - Share • As we go from left to right in period 2 first ionization energy actually decreases as we move from filling s to p block. Why? 76

Section 8. 7 Ions: Electron Configurations, Magnetic Properties, Ionic Radii and Ionization Energy Exceptions to Trends in First Ionization Energy Think – Pair - Share • As we go from left to right in period 2 first ionization from N to O decreases. Why? 77

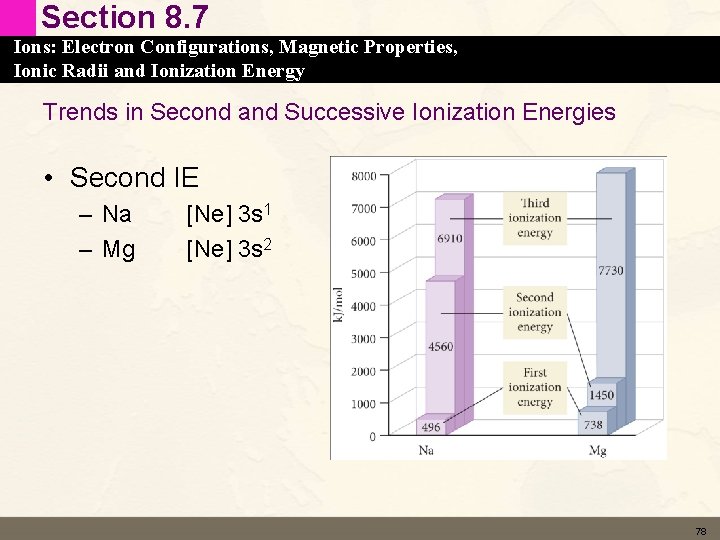
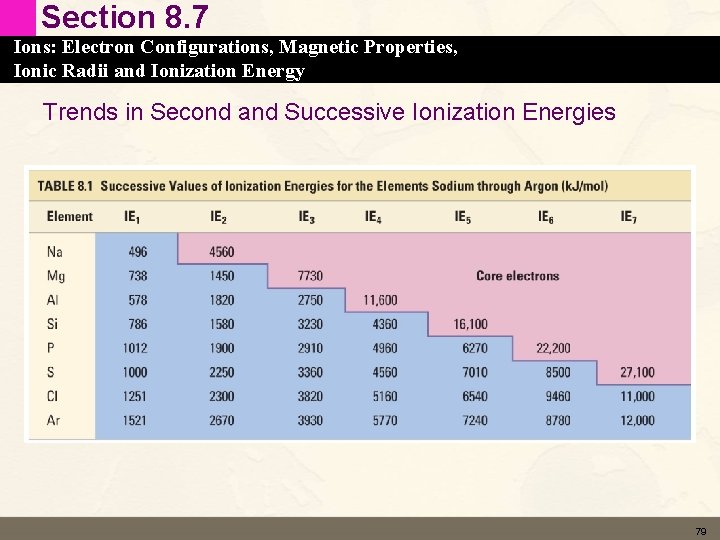
Section 8. 7 Ions: Electron Configurations, Magnetic Properties, Ionic Radii and Ionization Energy Trends in Second and Successive Ionization Energies • Second IE – Na – Mg [Ne] 3 s 1 [Ne] 3 s 2 78

Section 8. 7 Ions: Electron Configurations, Magnetic Properties, Ionic Radii and Ionization Energy Trends in Second and Successive Ionization Energies 79

Section 8. 8 Electron Affinities and Metallic Character Electron Affinity • Electron affinity is a measure of how easily an atom will accept an additional electron. • Can be measured as the energy change associated with the gaining of an electron is the gaseous state • Cl (g) + 1 e – Cl – (g) EA = – 349 k. J/mol • The more negative the value of EA the more readily an atom will accept an electron. 80

Section 8. 8 Electron Affinities and Metallic Character Trends in Electron Affinity • Most Groups do not have a regular trend in electron affinity • From left to right across a period electron affinities become more negative – More energy is released when an electron is added 81

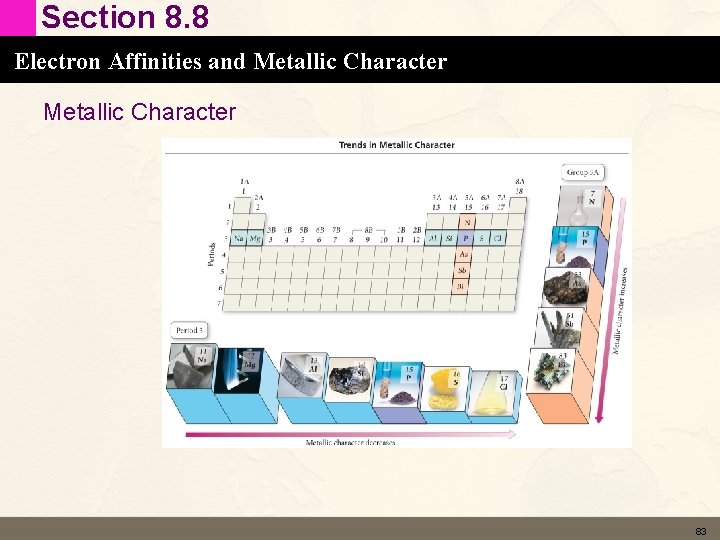
Section 8. 8 Electron Affinities and Metallic Character • • • Characteristics of Metals Good conductors of heat and electricity Malleable Ductile Shiny Lose electrons in chemical reactions 82

Section 8. 8 Electron Affinities and Metallic Character 83

Section 8. 9 Some Examples of Periodic Chemical Behavior The Alkali Metals (Group 1 A) • • • ns 1 outer electron configuration Low 1 st ionization energy Most reactive metals in the table Strong reducing agents IE decreases so reactivity increases down the group • 2 M + n. X 2 2 MXn • 2 M (s) + 2 H 2 O (l) 2 M+ (aq) + 2 OH – (aq) + H 2 (g) 84

Section 8. 9 Some Examples of Periodic Chemical Behavior The Halogens (Group 7 A) • • • ns 2 np 5 outer electron configuration Highly negative electron affinities Most active nonmetals in the table Powerful oxidizing agents IE decreases so reactivity increases down the group • 2 M + n. X 2 2 MXn • H 2 (g) + X 2 (g) 2 HX (g) • Br 2 (l) + F 2 (g) 2 Br. F (g) 85

Section 8. 9 Some Examples of Periodic Chemical Behavior The Noble Gases (Group 8 A) • • ns 2 np 6 outer electron configuration Full outer principle quantum level Most chemically inert Can be forced to react under extreme conditions Kr + F 2 Kr. F 2 Xe + F 2 Xe. F 2 Xe + O 2 Xe. O 2 86