Chapter 5 Gases AP CHEMISTRY Characteristics of Gases






















































- Slides: 85

Chapter 5 Gases AP CHEMISTRY

Characteristics of Gases • Gases are highly compressible and occupy the full volume of their containers. • When a gas is subjected to pressure, its volume decreases. • Gases always form homogeneous mixtures with other gases. • Gas molecules only occupy about 0. 1 % of the volume of their containers.


Pressure • Pressure is the force acting on an object per unit area: • Gravity exerts a force on the earth’s atmosphere • A column of air 1 m 2 in cross section exerts a force of 105 N. • The pressure of a 1 m 2 column of air is 100 k. Pa.


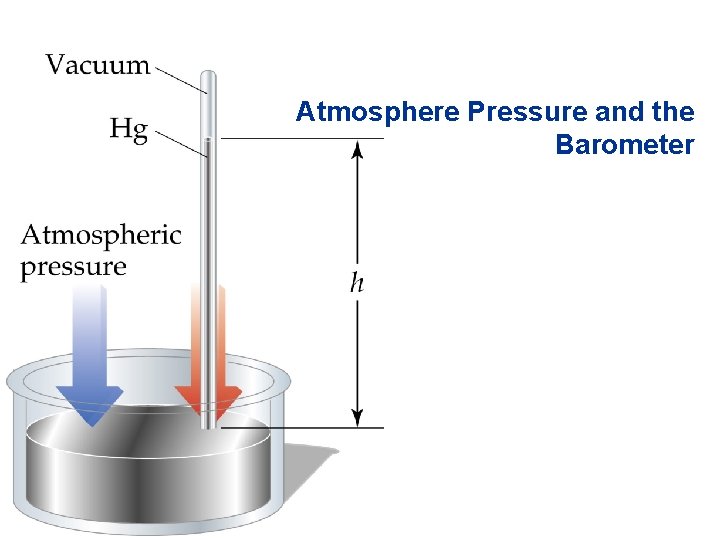
• • • Atmosphere Pressure and the Barometer SI Units: 1 N = 1 kg. m/s 2; 1 Pa = 1 N/m 2. Atmospheric pressure is measured with a barometer. If a vacated tube is inserted into a container of mercury open to the atmosphere, the mercury will rise 760 mm up the tube. Standard atmospheric pressure is the pressure required to support 760 mm of Hg in a column. Units: 1 atm = 760 mm. Hg = 760 torr = 1. 01325 105 Pa = 101. 325 k. Pa. = 14. 7 psi

Examples – Convert the following pressures: 1. 658. 2 mm Hg to k. Pa 87. 75 k. Pa

Examples – Convert the following pressures: 2. 1. 85 atm to torr 1410 torr

Examples – Convert the following pressures: 3. 337. 3 k. Pa to atm 3. 329 atm

Atmosphere Pressure and the Barometer

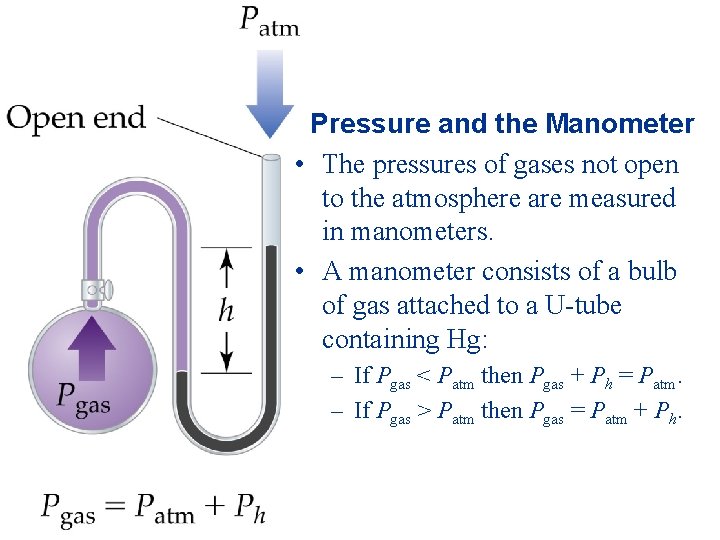
Pressure and the Manometer • The pressures of gases not open to the atmosphere are measured in manometers. • A manometer consists of a bulb of gas attached to a U-tube containing Hg: – If Pgas < Patm then Pgas + Ph = Patm. – If Pgas > Patm then Pgas = Patm + Ph.

Example – The mercury in a manometer is 46 mm higher on the open end than on the gas bulb end. If atmospheric pressure is 102. 2 k. Pa, what is the pressure of the gas in the bulb?

Example – The mercury in a manometer is 46 mm higher on the open end than on the gas bulb end. If atmospheric pressure is 102. 2 k. Pa, what is the pressure of the gas in the bulb? 813 mm Hg 108 k. Pa

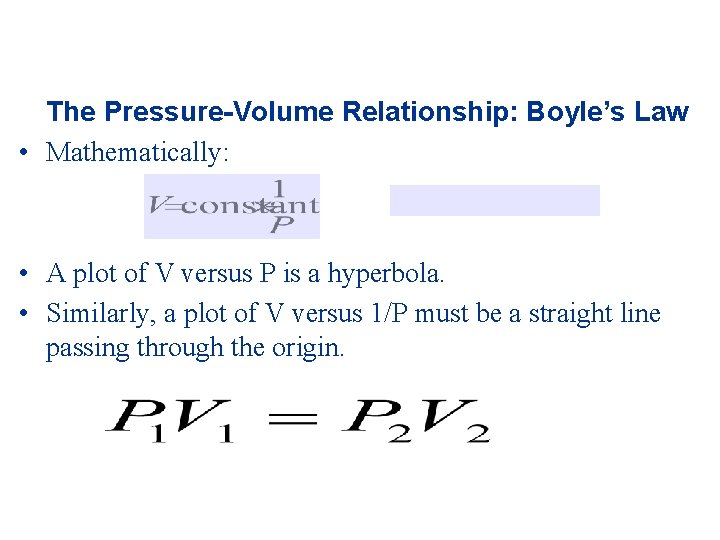
The Gas Laws • • • The Pressure-Volume Relationship: Boyle’s Law Weather balloons are used as a practical consequence to the relationship between pressure and volume of a gas. As the weather balloon ascends, the volume increases. As the weather balloon gets further from the earth’s surface, the atmospheric pressure decreases. Boyle’s Law: the volume of a fixed quantity of gas is inversely proportional to its pressure (assuming all other variables are unchanged). Boyle used a manometer to carry out the experiment.


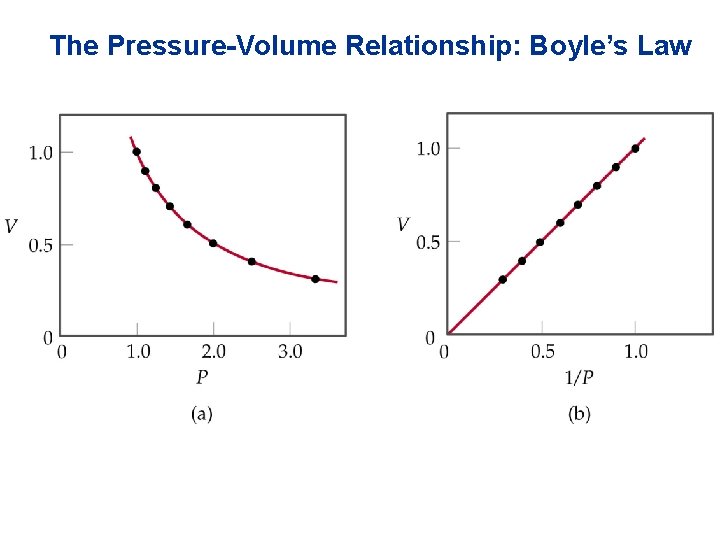
The Pressure-Volume Relationship: Boyle’s Law • Mathematically: • A plot of V versus P is a hyperbola. • Similarly, a plot of V versus 1/P must be a straight line passing through the origin.

The Pressure-Volume Relationship: Boyle’s Law

Boyle’s Law Examples 1. A gas that occupies 2. 84 L has a pressure of 88. 6 k. Pa. What would be the pressure of the gas sample if it only occupied 1. 66 L (assuming the same temperature)?

Boyle’s Law Examples 1. A gas that occupies 2. 84 L has a pressure of 88. 6 k. Pa. What would be the pressure of the gas sample if it only occupied 1. 66 L (assuming the same temperature)? 2. 152 k. Pa

Boyle’s Law Examples 2. A 10. 0 -L sample of argon gas has a pressure of 0. 885 atm. At what volume would the sample have a pressure of 6. 72 atm?

Boyle’s Law Examples 2. A 10. 0 -L sample of argon gas has a pressure of 0. 885 atm. At what volume would the sample have a pressure of 6. 72 atm? 1. 32 L

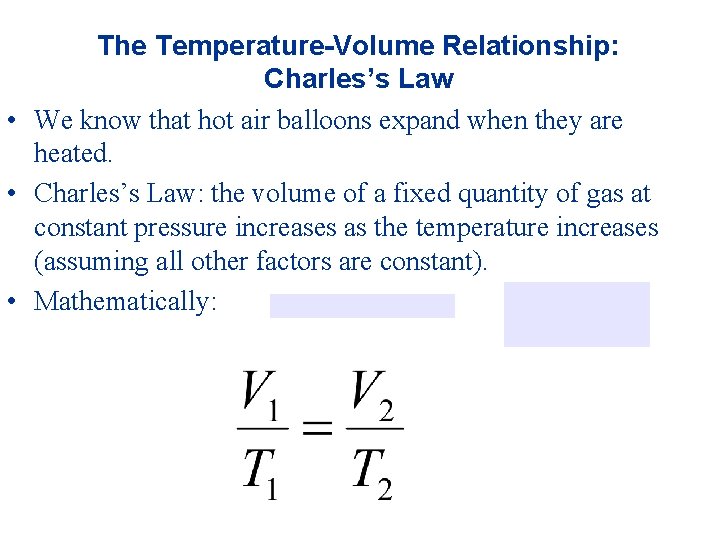
The Temperature-Volume Relationship: Charles’s Law • We know that hot air balloons expand when they are heated. • Charles’s Law: the volume of a fixed quantity of gas at constant pressure increases as the temperature increases (assuming all other factors are constant). • Mathematically:


• A plot of V versus T is a straight line. • When T is measured in C, the intercept on the temperature axis is -273. 15 C. • We define absolute zero, 0 K = -273. 15 C. • Note the value of the constant reflects the assumptions: amount of gas and pressure.

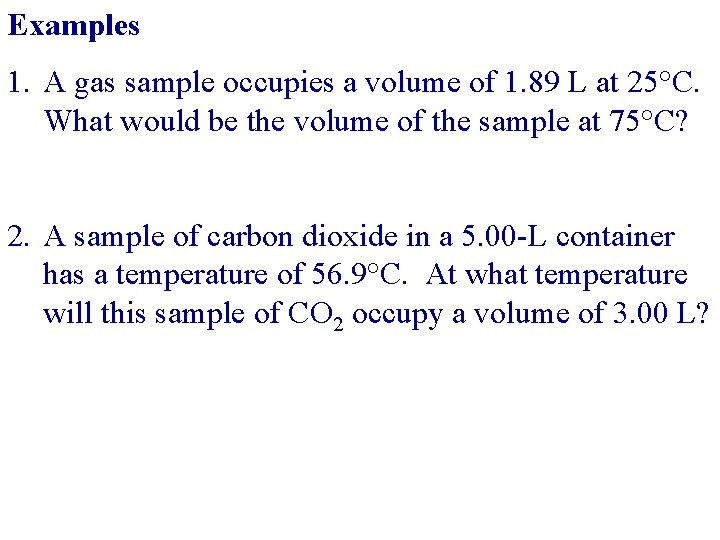
Examples 1. A gas sample occupies a volume of 1. 89 L at 25°C. What would be the volume of the sample at 75°C? 2. A sample of carbon dioxide in a 5. 00 -L container has a temperature of 56. 9°C. At what temperature will this sample of CO 2 occupy a volume of 3. 00 L?

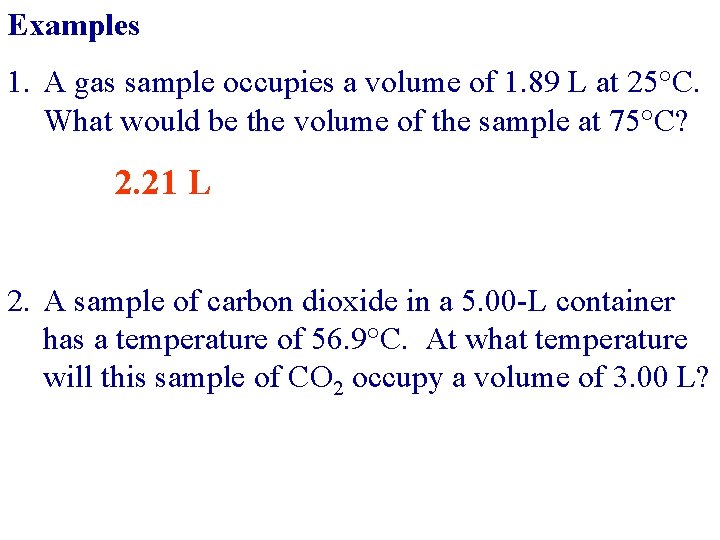
Examples 1. A gas sample occupies a volume of 1. 89 L at 25°C. What would be the volume of the sample at 75°C? 2. 21 L 2. A sample of carbon dioxide in a 5. 00 -L container has a temperature of 56. 9°C. At what temperature will this sample of CO 2 occupy a volume of 3. 00 L?

Examples 1. A gas sample occupies a volume of 1. 89 L at 25°C. What would be the volume of the sample at 75°C? 2. 21 L 2. A sample of carbon dioxide in a 5. 00 -L container has a temperature of 56. 9°C. At what temperature will this sample of CO 2 occupy a volume of 3. 00 L?

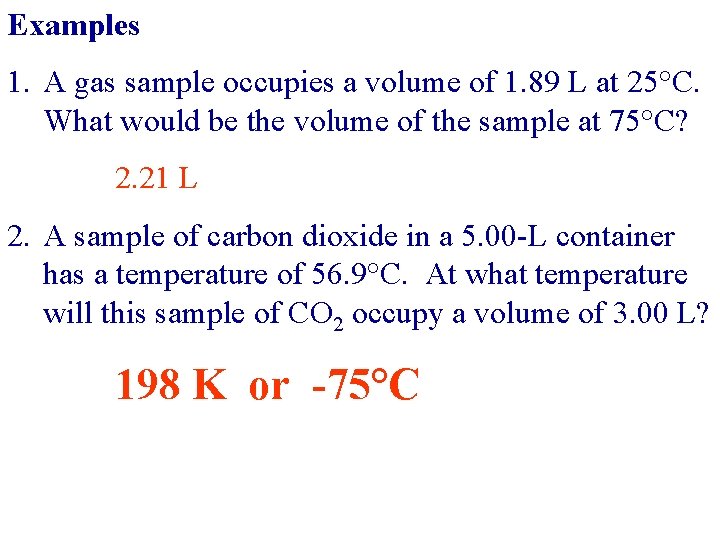
Examples 1. A gas sample occupies a volume of 1. 89 L at 25°C. What would be the volume of the sample at 75°C? 2. 21 L 2. A sample of carbon dioxide in a 5. 00 -L container has a temperature of 56. 9°C. At what temperature will this sample of CO 2 occupy a volume of 3. 00 L? 198 K or -75°C

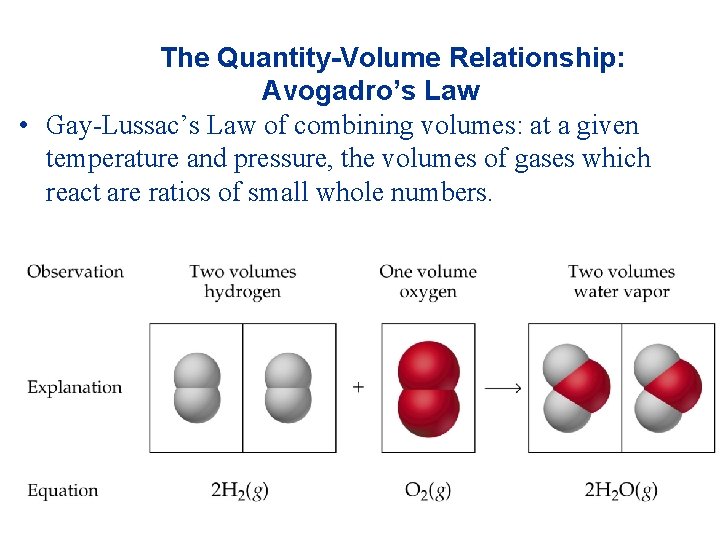
The Quantity-Volume Relationship: Avogadro’s Law • Gay-Lussac’s Law of combining volumes: at a given temperature and pressure, the volumes of gases which react are ratios of small whole numbers.

• Avogadro’s Hypothesis: equal volumes of gas at the same temperature and pressure will contain the same number of molecules. • Avogadro’s Law: the volume of gas at a given temperature and pressure is directly proportional to the number of moles of gas.

• Mathematically: • We can show that 22. 4 L of any gas at 0 C contain 6. 02 1023 gas molecules.


The Ideal Gas Equation • Consider the three gas laws. • Boyle’s Law: • Charles’s Law: • Avogadro’s Law: • We can combine these into a general gas law:

The Ideal Gas Equation • If R is the constant of proportionality (called the gas constant), then • The ideal gas equation is: • R = 0. 08206 L·atm/mol·K = 8. 314 J/mol·K


• We define STP (standard temperature and pressure) = 0 C, 273. 15 K, 1 atm. • Volume of 1 mol of gas at STP is:

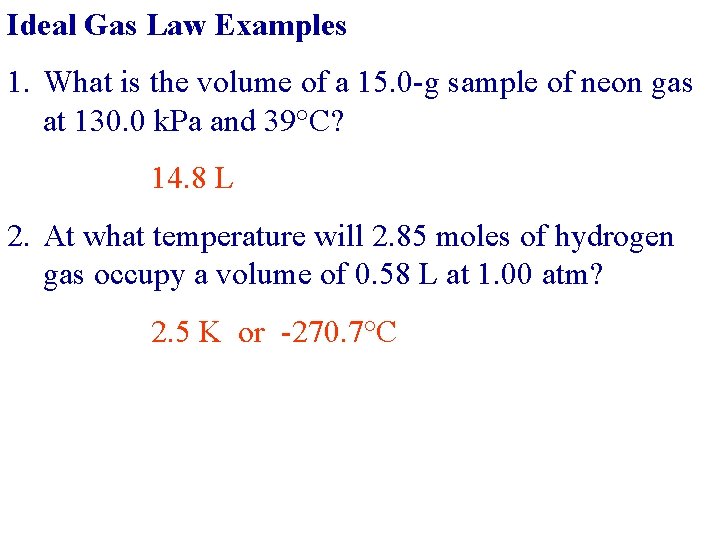
Ideal Gas Law Examples 1. What is the volume of a 15. 0 -g sample of neon gas at 130. 0 k. Pa and 39°C? 14. 8 L 2. At what temperature will 2. 85 moles of hydrogen gas occupy a volume of 0. 58 L at 1. 00 atm? 2. 5 K or -270. 7°C

Relating the Ideal-Gas Equation and the Gas Laws • If PV = n. RT and n and T are constant, then PV = constant and we have Boyle’s law. • Other laws can be generated similarly. • In general, if we have a gas under two sets of conditions, then

Examples 1. A gas sample occupies a volume of 0. 685 L at 38°C and 0. 775 atm. What will be the temperature of the sample if it occupies 0. 125 L at 1. 25 atm? 91. 6 K or -181. 6°C 2. A sample of nitrogen gas in a 2. 00 L container has a pressure of 1800. 6 mm. Hg at 25°C. What would be the pressure on the sample in a 1. 00 L container at 125°C? 4810 mm Hg

Examples 1. A gas sample occupies a volume of 0. 685 L at 38°C and 0. 775 atm. What will be the temperature of the sample if it occupies 0. 125 L at 1. 25 atm? 91. 6 K or -181. 6°C 2. A sample of nitrogen gas in a 2. 00 L container has a pressure of 1800. 6 mm. Hg at 25°C. What would be the pressure on the sample in a 1. 00 L container at 125°C? 4810 mm Hg

Examples 1. A gas sample occupies a volume of 0. 685 L at 38°C and 0. 775 atm. What will be the temperature of the sample if it occupies 0. 125 L at 1. 25 atm? 91. 6 K or -181. 6°C 2. A sample of nitrogen gas in a 2. 00 L container has a pressure of 1800. 6 mm. Hg at 25°C. What would be the pressure on the sample in a 1. 00 L container at 125°C? 4810 mm Hg

Further Applications of the Ideal-Gas Equation Gas Densities and Molar Mass • Density has units of mass over volume. • Rearranging the ideal-gas equation with M as molar mass we get DO NOT memorize this! Better equations On the next slide!

Further Applications of the Ideal-Gas Equation Gas Densities and Molar Mass • Density has units of mass over volume. • Using simple definitions we get Zygas Approved!

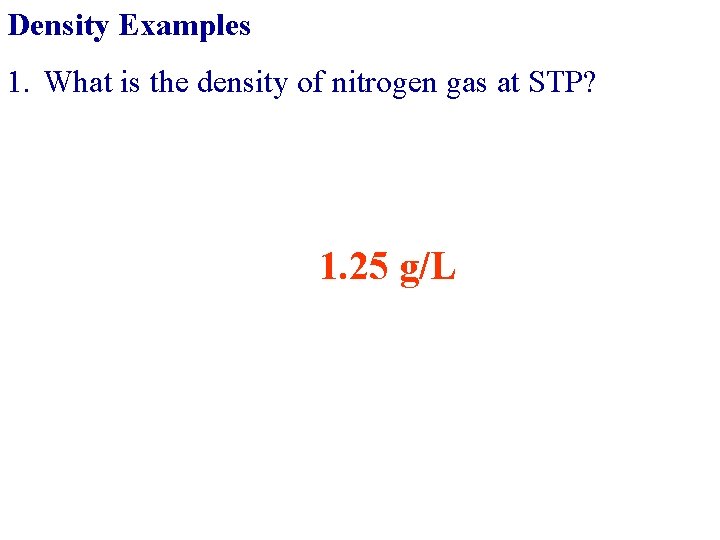
Density Examples 1. What is the density of nitrogen gas at STP?

Density Examples 1. What is the density of nitrogen gas at STP? 1. 25 g/L

Density Examples 2. What is the density of nitrogen gas at 35°C and 1. 50 atm?

Density Examples 2. What is the density of nitrogen gas at 35°C and 1. 50 atm? 1. 67 g/L

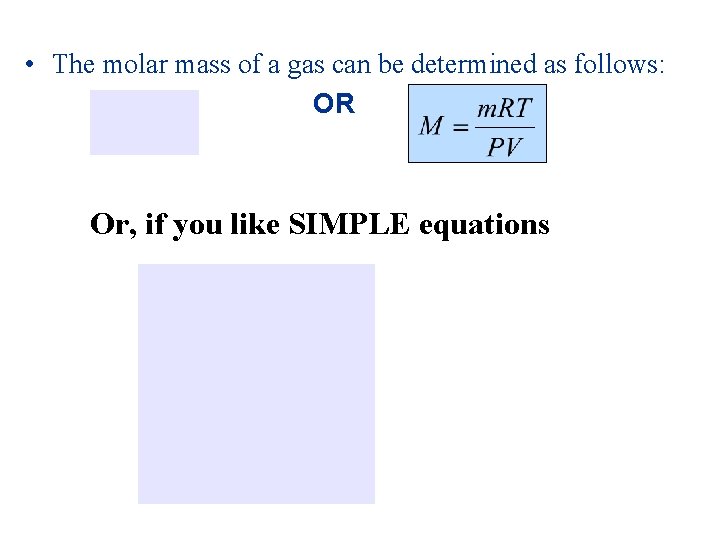
• The molar mass of a gas can be determined as follows: OR Or, if you like SIMPLE equations

Volumes of Gases in Chemical Reactions • The ideal-gas equation relates P, V, and T to number of moles of gas. • The n can then be used in stoichiometric calculations.

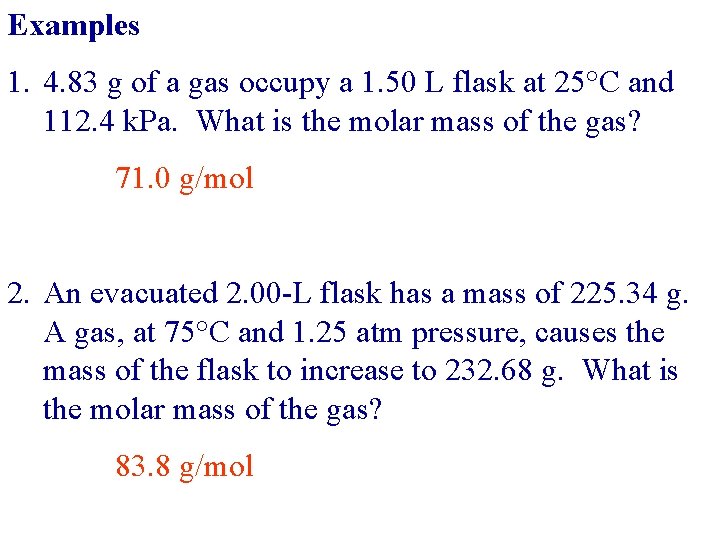
Examples 1. 4. 83 g of a gas occupy a 1. 50 L flask at 25°C and 112. 4 k. Pa. What is the molar mass of the gas? 71. 0 g/mol 2. An evacuated 2. 00 -L flask has a mass of 225. 34 g. A gas, at 75°C and 1. 25 atm pressure, causes the mass of the flask to increase to 232. 68 g. What is the molar mass of the gas? 83. 8 g/mol

Gas Mixtures and Partial Pressures • Since gas molecules are so far apart, we can assume they behave independently. • Dalton’s Law: in a gas mixture the total pressure is given by the sum of partial pressures of each component: • Each gas obeys the ideal gas equation:

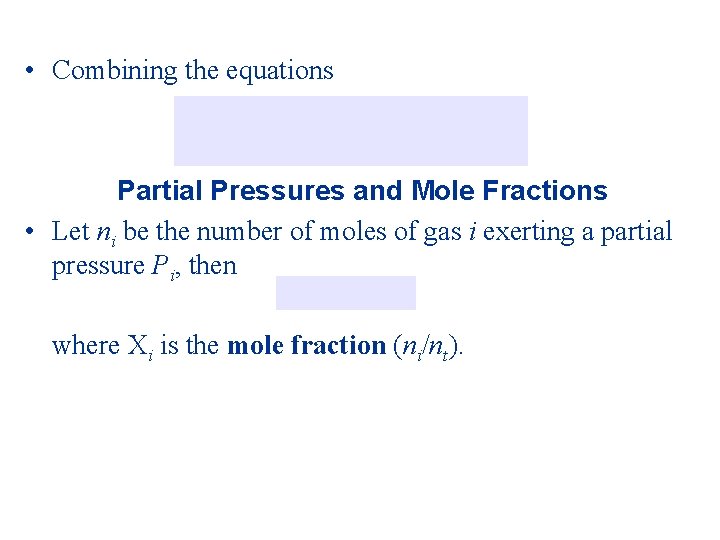
• Combining the equations Partial Pressures and Mole Fractions • Let ni be the number of moles of gas i exerting a partial pressure Pi, then where i is the mole fraction (ni/nt).

Examples: 1. Hydrogen gas is added to a 2. 00 -L flask at a pressure of 5. 6 atm. Oxygen gas is added until the total pressure in the flask measures 8. 4 atm. What is the mole fraction of hydrogen in the flask? 2. 1. 35 moles of argon and 2. 75 moles of neon are placed in a 15. 0 -L tank at 35°C. What is the total pressure in the flask? What is the pressure exerted by neon?

Examples: 1. Hydrogen gas is added to a 2. 00 -L flask at a pressure of 5. 6 atm. Oxygen gas is added until the total pressure in the flask measures 8. 4 atm. What is the mole fraction of hydrogen in the flask? 0. 67 2. 1. 35 moles of argon and 2. 75 moles of neon are placed in a 15. 0 -L tank at 35°C. What is the total pressure in the flask? What is the pressure exerted by neon? Ptot = 6. 91 atm PNe = 4. 63 atm

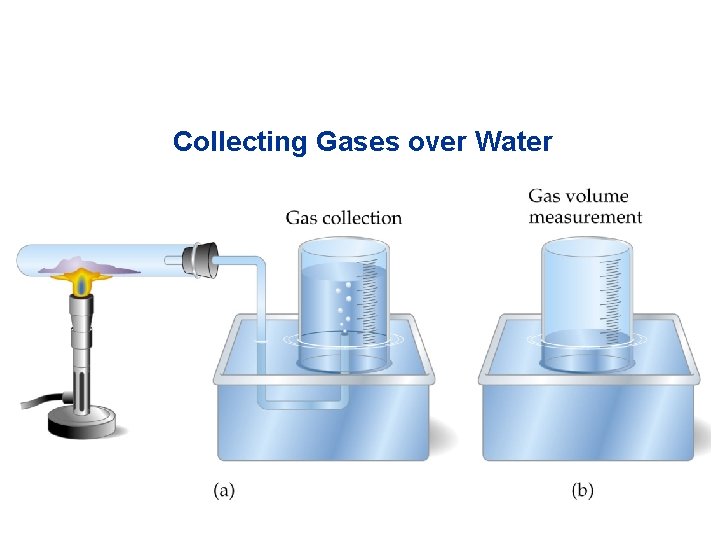
Collecting Gases over Water • It is common to synthesize gases and collect them by displacing a volume of water. • To calculate the amount of gas produced, we need to correct for the partial pressure of the water:

Collecting Gases over Water

Example - 150. 82 m. L of an unknown gas is collected over water at 27°C and 1. 032 atm. The mass of the gas is 0. 1644 g. What is the molar mass of the gas? The vapor pressure of water at 27°C is 26. 74 torr

Kinetic Molecular Theory • Theory developed to explain gas behavior. • Theory of moving molecules. • Assumptions: – Gases consist of a large number of molecules in constant random motion. – Volume of individual molecules negligible compared to volume of container. – Intermolecular forces (forces between gas molecules) negligible.

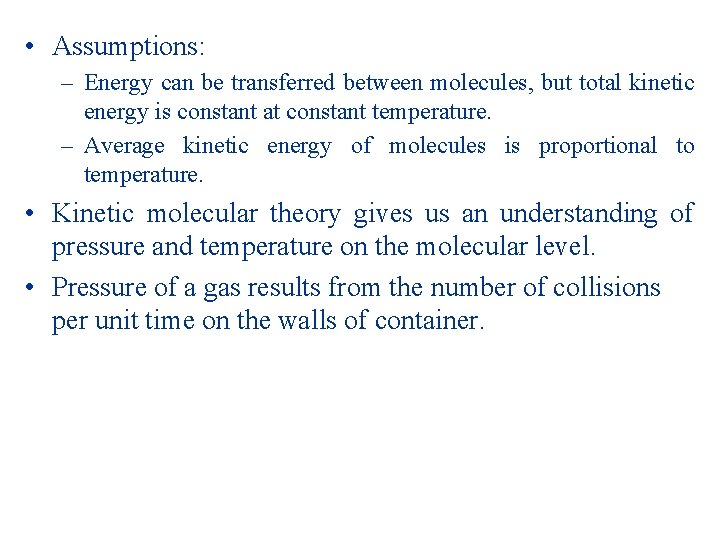
• Assumptions: – Energy can be transferred between molecules, but total kinetic energy is constant at constant temperature. – Average kinetic energy of molecules is proportional to temperature. • Kinetic molecular theory gives us an understanding of pressure and temperature on the molecular level. • Pressure of a gas results from the number of collisions per unit time on the walls of container.

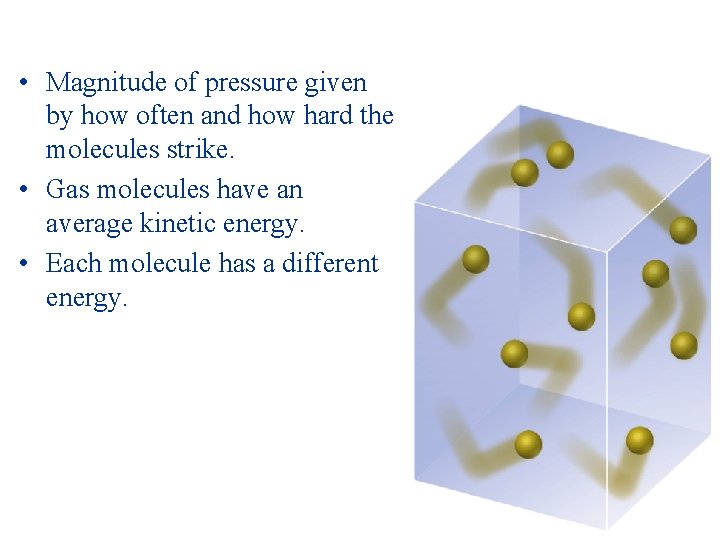
• Magnitude of pressure given by how often and how hard the molecules strike. • Gas molecules have an average kinetic energy. • Each molecule has a different energy.

• There is a spread of individual energies of gas molecules in any sample of gas. • As the temperature increases, the average kinetic energy of the gas molecules increases.


• As kinetic energy increases, the velocity of the gas molecules increases. • Root mean square speed, u, is the speed of a gas molecule having average kinetic energy. • Average kinetic energy, , is related to root mean square speed:

Application to Gas Laws • As volume increases at constant temperature, the average kinetic energy of the gas remains constant. Therefore, u is constant. However, volume increases so the gas molecules have to travel further to hit the walls of the container. Therefore, pressure decreases. • If temperature increases at constant volume, the average kinetic energy of the gas molecules increases. Therefore, there are more collisions with the container walls and the pressure increases.

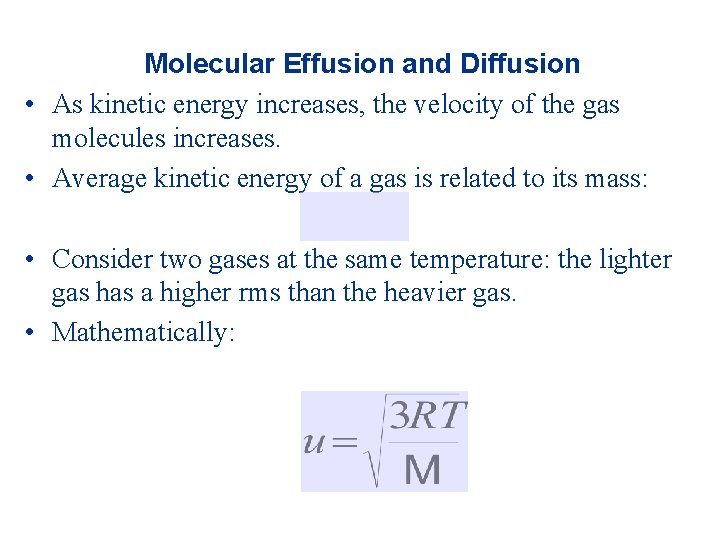
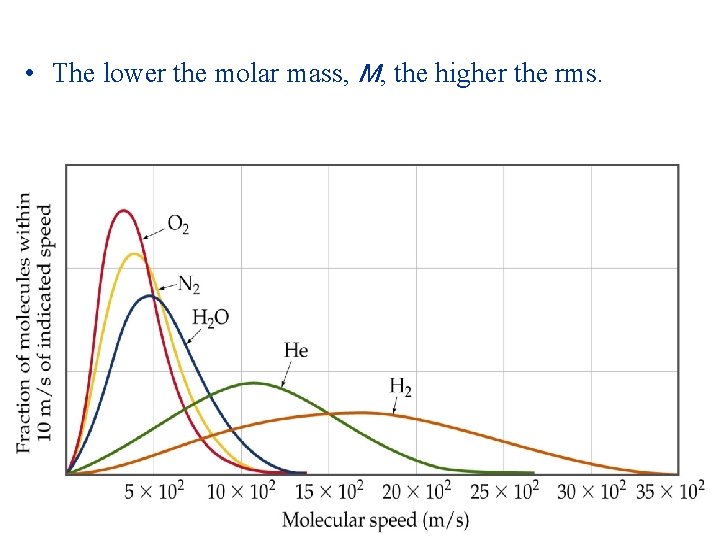
Molecular Effusion and Diffusion • As kinetic energy increases, the velocity of the gas molecules increases. • Average kinetic energy of a gas is related to its mass: • Consider two gases at the same temperature: the lighter gas has a higher rms than the heavier gas. • Mathematically:

• The lower the molar mass, M, the higher the rms.

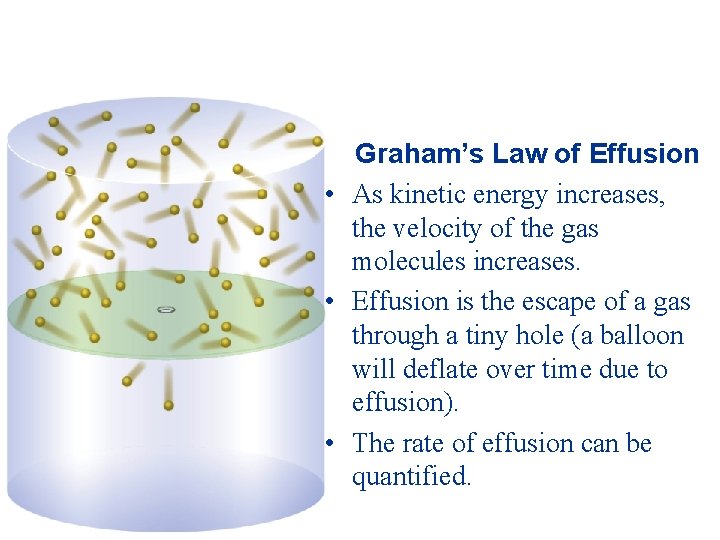
Graham’s Law of Effusion • As kinetic energy increases, the velocity of the gas molecules increases. • Effusion is the escape of a gas through a tiny hole (a balloon will deflate over time due to effusion). • The rate of effusion can be quantified.

• Consider two gases with molar masses M 1 and M 2, the relative rate of effusion is given by: • Only those molecules that hit the small hole will escape through it. • Therefore, the higher the rms the more likelihood of a gas molecule hitting the hole.

• Consider two gases with molar masses M 1 and M 2, the relative rate of effusion is given by: • Only those molecules that hit the small hole will escape through it. • Therefore, the higher the rms the more likelihood of a gas molecule hitting the hole.

Examples – For each pair of gases, determine which will effuse faster, and by how much it will be faster. 1. CH 4 and Xe 2. Cl 2 and N 2 3. F 2 and He

Examples – For each pair of gases, determine which will effuse faster, and by how much it will be faster. 1. CH 4 and Xe 2. 8607 2. Cl 2 and N 2 1. 59095 3. F 2 and He 3. 08027

• • Diffusion and Mean Free Path Diffusion of a gas is the spread of the gas through space. Diffusion is faster for light gas molecules. Diffusion is significantly slower than rms speed (consider someone opening a perfume bottle: it takes while to detect the odor but rms speed at 25 C is about 1150 mi/hr). Diffusion is slowed by gas molecules colliding with each other.

• Average distance of a gas molecule between collisions is called mean free path. • At sea level, mean free path is about 6 10 -6 cm.

Real Gases: Deviations from Ideal Behavior • From the ideal gas equation, we have • For 1 mol of gas, PV/RT = 1 for all pressures. • In a real gas, PV/RT varies from 1 significantly. • The higher the pressure the more the deviation from ideal behavior.


• From the ideal gas equation, we have • For 1 mol of gas, PV/RT = 1 for all temperatures. • As temperature increases, the gases behave more ideally. • The assumptions in kinetic molecular theory show where ideal gas behavior breaks down: – the molecules of a gas have finite volume; – molecules of a gas do attract each other.


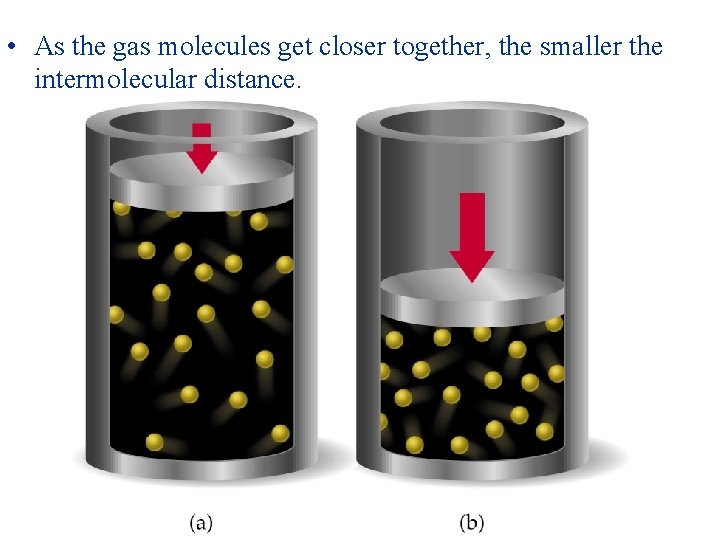
• As the pressure on a gas increases, the molecules are forced closer together. • As the molecules get closer together, the volume of the container gets smaller. • The smaller the container, the more space the gas molecules begin to occupy. • Therefore, the higher the pressure, the less the gas resembles an ideal gas.

• As the gas molecules get closer together, the smaller the intermolecular distance.

• The smaller the distance between gas molecules, the more likely attractive forces will develop between the molecules. • Therefore, the less the gas resembles and ideal gas. • As temperature increases, the gas molecules move faster and further apart. • Also, higher temperatures mean more energy available to break intermolecular forces.

Real Gases: Deviations from Ideal Behavior • Therefore, the higher the temperature, the more ideal the gas.

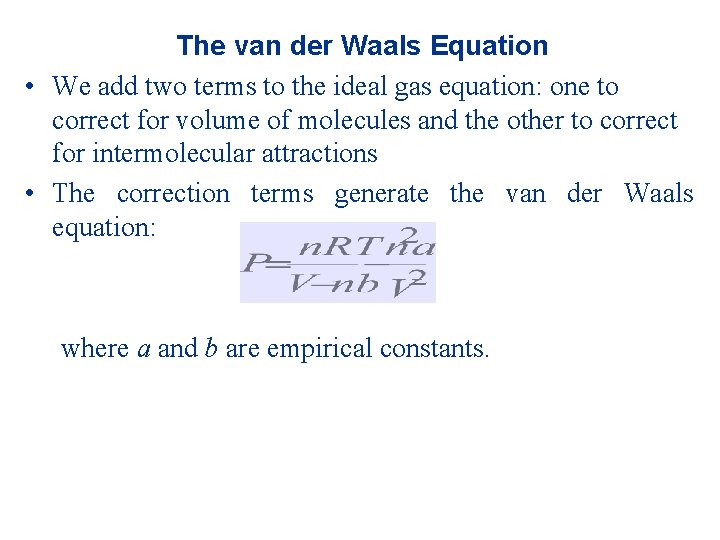
The van der Waals Equation • We add two terms to the ideal gas equation: one to correct for volume of molecules and the other to correct for intermolecular attractions • The correction terms generate the van der Waals equation: where a and b are empirical constants.

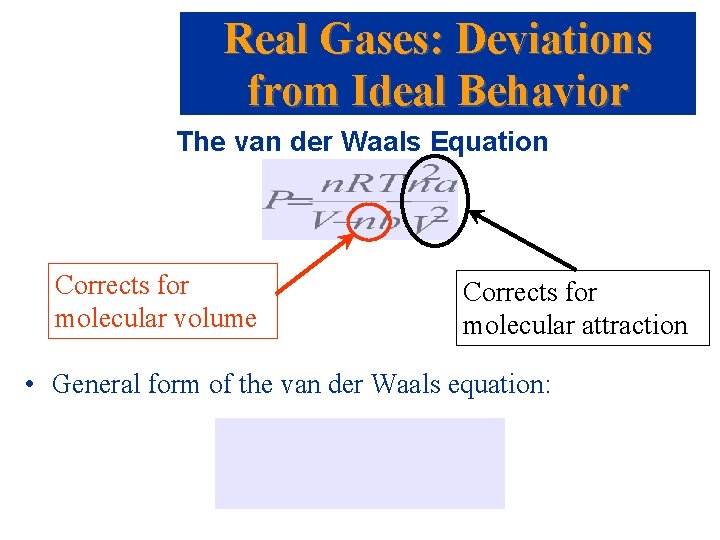
Real Gases: Deviations from Ideal Behavior The van der Waals Equation Corrects for molecular volume Corrects for molecular attraction • General form of the van der Waals equation:


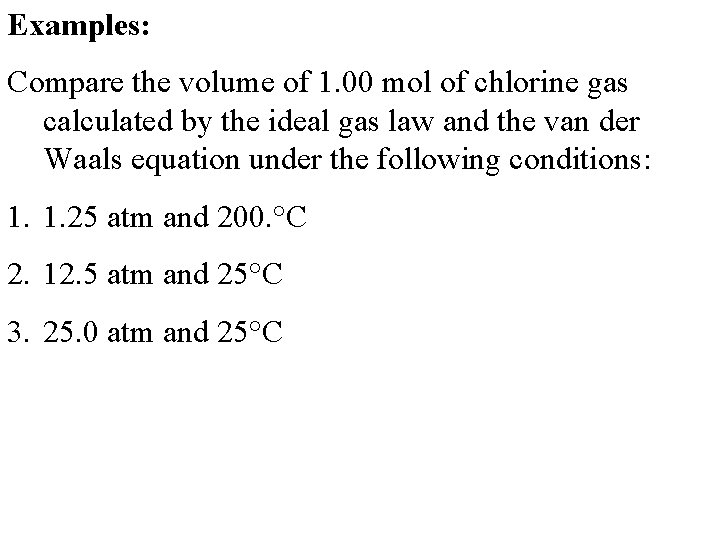
Examples: Compare the volume of 1. 00 mol of chlorine gas calculated by the ideal gas law and the van der Waals equation under the following conditions: 1. 1. 25 atm and 200. °C 2. 12. 5 atm and 25°C 3. 25. 0 atm and 25°C
 Chemistry chapter 11 gases test
Chemistry chapter 11 gases test Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Characteristics of gases
Characteristics of gases Characteristics of noble gas
Characteristics of noble gas What are the different properties of gas?
What are the different properties of gas? Gases characteristics
Gases characteristics Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Characteristics of ideal gases
Characteristics of ideal gases Ideal gas characteristics
Ideal gas characteristics Chapter 11 review gases section 1
Chapter 11 review gases section 1 How to solve ideal gas law
How to solve ideal gas law Chapter 14 the behavior of gases
Chapter 14 the behavior of gases Which gas law relates pressure and temperature
Which gas law relates pressure and temperature Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kinetic molecular theory of solid
Kinetic molecular theory of solid Is chalk natural or manmade
Is chalk natural or manmade Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Organic vs inorganic compounds
Organic vs inorganic compounds Chapter 9 review stoichiometry
Chapter 9 review stoichiometry Love formula
Love formula Modern chemistry chapter 15
Modern chemistry chapter 15 Chapter 14 review acids and bases
Chapter 14 review acids and bases Modern chemistry chapter 13 review answers
Modern chemistry chapter 13 review answers Chapter 12 review solutions section 1
Chapter 12 review solutions section 1 Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 study guide the mole
Chapter 10 study guide the mole Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Chapter 7 chemistry review
Chapter 7 chemistry review Radioactive tracers in agriculture
Radioactive tracers in agriculture Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Chapter 12 basics of chemistry cosmetology
Chapter 12 basics of chemistry cosmetology Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Organic chemistry (3rd) edition chapter 1 problem 20s
Organic chemistry (3rd) edition chapter 1 problem 20s Modern chemistry chapter 4
Modern chemistry chapter 4 Chemistry central science 14th edition
Chemistry central science 14th edition Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chapter 11 study guide chemistry stoichiometry answer key
Chapter 11 study guide chemistry stoichiometry answer key Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key Chapter 1 introduction to chemistry
Chapter 1 introduction to chemistry Chapter 13 mixtures and solutions answers
Chapter 13 mixtures and solutions answers Chapter 9 stoichiometry test answer key
Chapter 9 stoichiometry test answer key Chemistry matter and change answer key chapter 2
Chemistry matter and change answer key chapter 2 Ap chemistry chapter 11
Ap chemistry chapter 11 Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp Introduction to chemistry chapter 1
Introduction to chemistry chapter 1 3 scientific measurement
3 scientific measurement Inorganic pharmaceutical
Inorganic pharmaceutical Ap chemistry chapter 7 periodic properties of the elements
Ap chemistry chapter 7 periodic properties of the elements Organic chemistry chapter 9
Organic chemistry chapter 9 Chemistry 101 chapter 1
Chemistry 101 chapter 1 Ap chemistry chapter 8
Ap chemistry chapter 8 Chapter 6 chemistry in biology
Chapter 6 chemistry in biology Non examples of homogeneous mixture
Non examples of homogeneous mixture Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Basic food chemistry the nature of matter
Basic food chemistry the nature of matter This name
This name Chapter 2 the chemistry of life section review 2-2
Chapter 2 the chemistry of life section review 2-2 Oxygen periodic trends
Oxygen periodic trends Chapter 6 ap chemistry
Chapter 6 ap chemistry Chemistry in biology chapter 6 section 1 answer key
Chemistry in biology chapter 6 section 1 answer key Chapter 2 chemistry comes alive answer key
Chapter 2 chemistry comes alive answer key Chapter 2 essential chemistry for biology
Chapter 2 essential chemistry for biology Halohydrin
Halohydrin Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Anatomy and physiology chapter 2
Anatomy and physiology chapter 2 Chapter 8 test review chemistry
Chapter 8 test review chemistry 7 ionic and metallic bonding practice problems
7 ionic and metallic bonding practice problems Chapter 6 section 3 water and solutions
Chapter 6 section 3 water and solutions Nuclear fusion
Nuclear fusion Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry Chapter 2 chemistry comes alive
Chapter 2 chemistry comes alive Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Chapter 10 nuclear chemistry
Chapter 10 nuclear chemistry Ap chemistry atomic structure and periodicity
Ap chemistry atomic structure and periodicity Ap chemistry chapter 5
Ap chemistry chapter 5 Sistema termodinamico
Sistema termodinamico What is boron
What is boron Thermal expansion and contraction examples
Thermal expansion and contraction examples