Topic Chemistry Aim Explain how elements are classified























- Slides: 49

Topic: Chemistry Aim: Explain how elements are classified in the Periodic Table of Elements. Do Now: next slide HW: Atoms, Elements, Compounds and Mixtures Exam Review Sheet

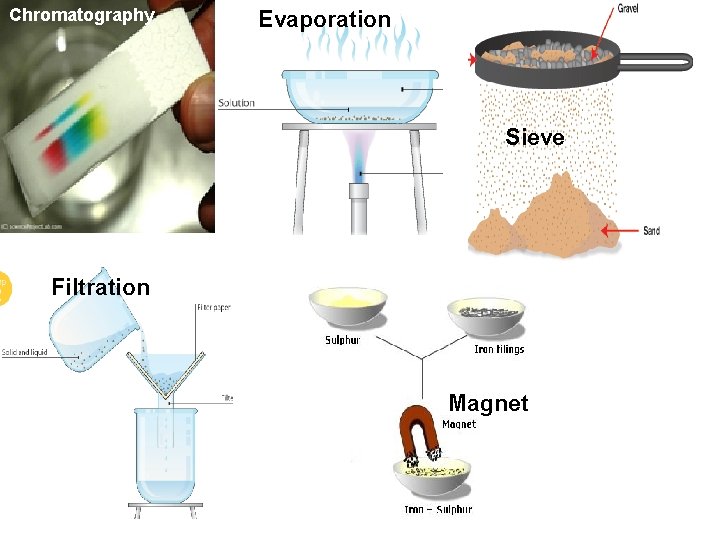
Chromatography Evaporation Sieve Filtration Magnet

The substances in a mixture can be separated by physical means because 1. the substances are chemically combined. 2. the substances are chemically conded together. 3. the substances are physically, not chemically, mixed.

Sand iron particles that are similar in size and color are mixed together in a beaker. What would be the best method of separating the particles? 1. Use tweezers to separate them. 2. Add water to the mixture. 3. Use a magnet to separate them. 4. Pour the mixture into a filter.

A green plant contains the green pigment chlorophyll. However, it also contains other pigments such as carotene and xanthophyll. How can these pigments be separated from each other? 1. Use tweezers to separate them. 2. Use filtration to separate them. 3. Use chromatography to separate them. 4. Use a sieve to separate them.

How can you separate sugar from a sugar water solution? 1. Use a magnet to separate the sugar from the water. 2. Use filtration to separate the sugar from the water. 3. Use chromatography to separate the sugar from the water. 4. Use evaporation to separate the sugar from the water.

How can you separate a solid from a liquid mixture? 1. Use a magnet to separate the insoluble substance from the mixture. 2. Use evaporation to separate the insoluble substance from the mixture 3. Use filtration to separate the insoluble substance from the mixture. 4. Use chromatography to separate the insoluble substance from the mixture.

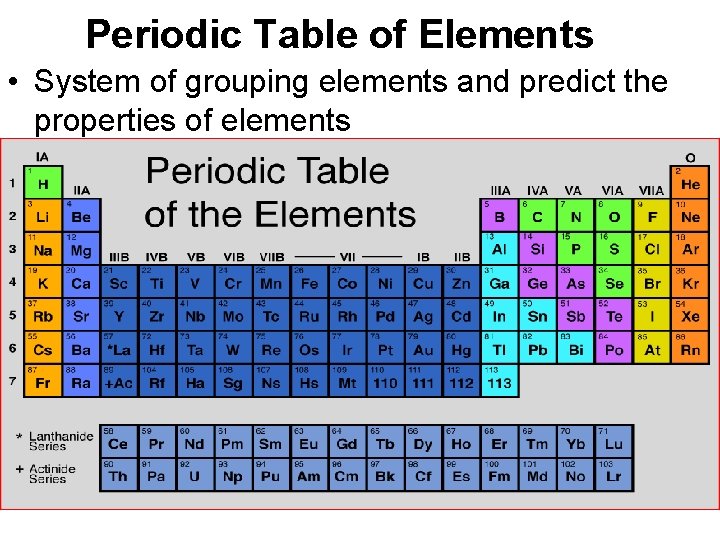
Periodic Table of Elements • System of grouping elements and predict the properties of elements

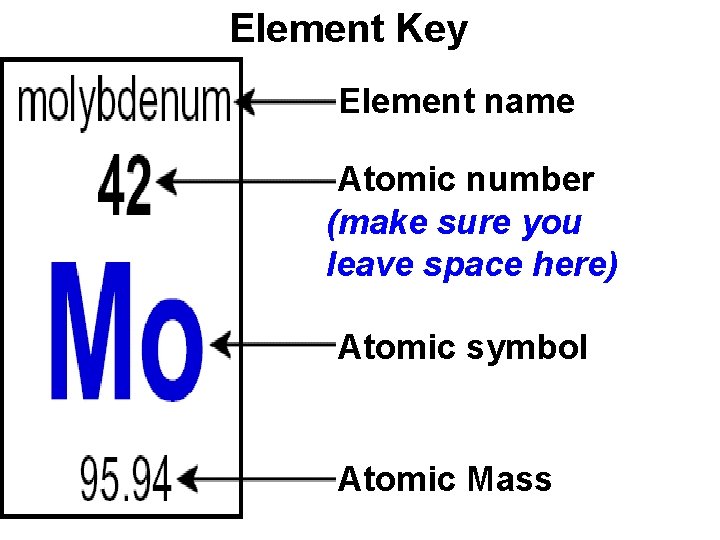
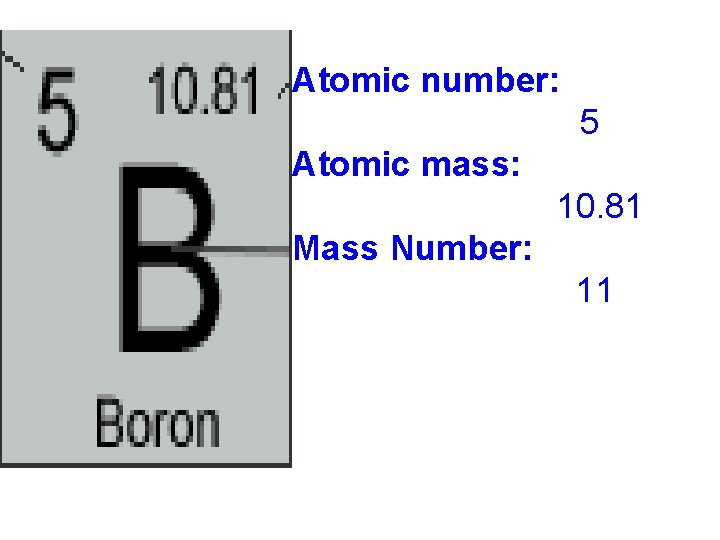
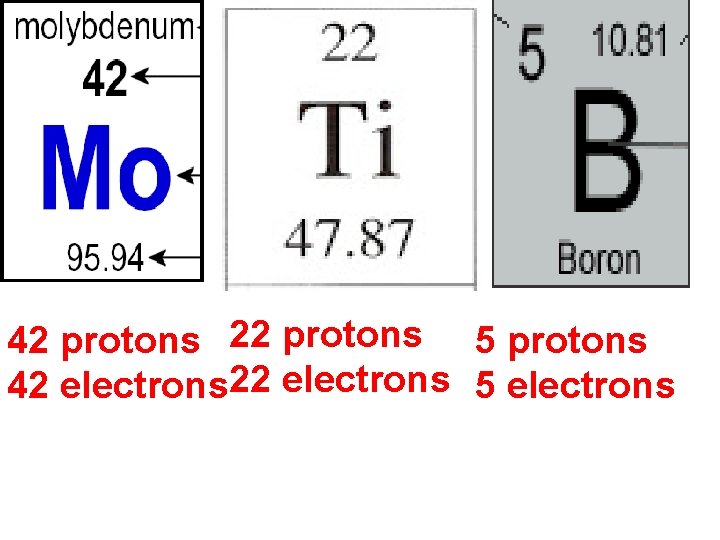
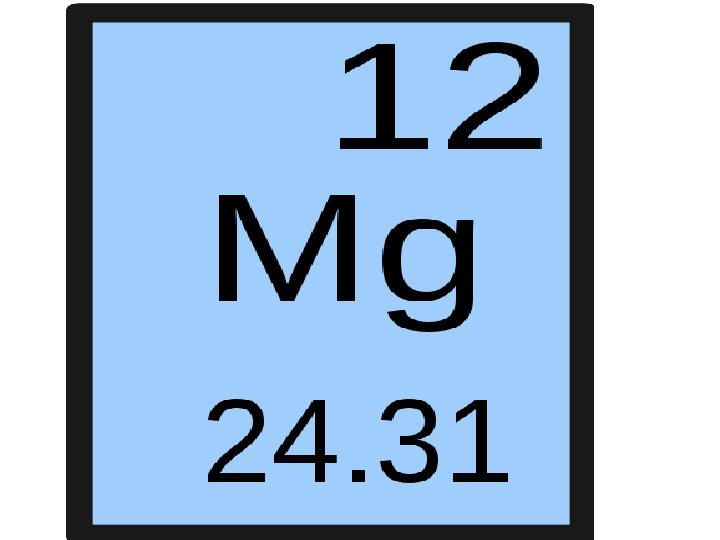
Element Key Element name Atomic number (make sure you leave space here) Atomic symbol Atomic Mass

Atomic number: • # of protons • Different for each element • Never changes

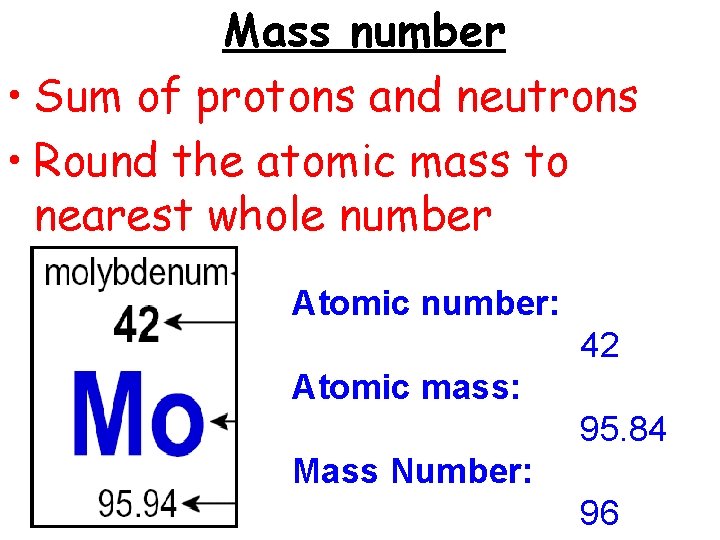
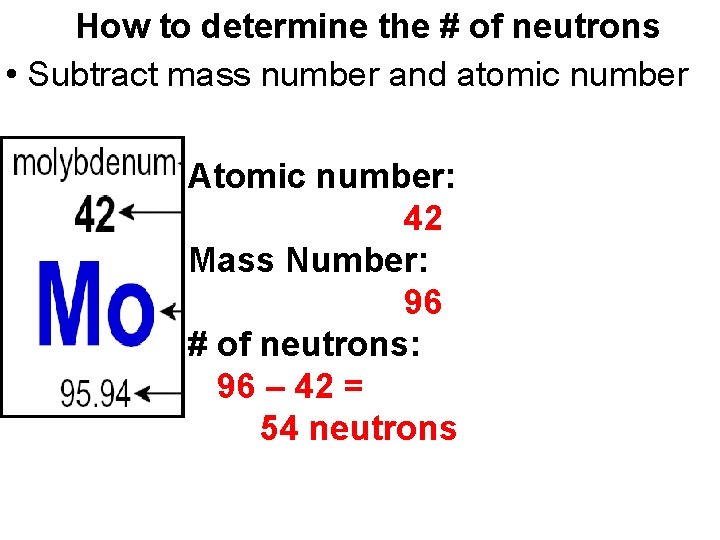
Mass number • Sum of protons and neutrons • Round the atomic mass to nearest whole number Atomic number: 42 Atomic mass: 95. 84 Mass Number: 96

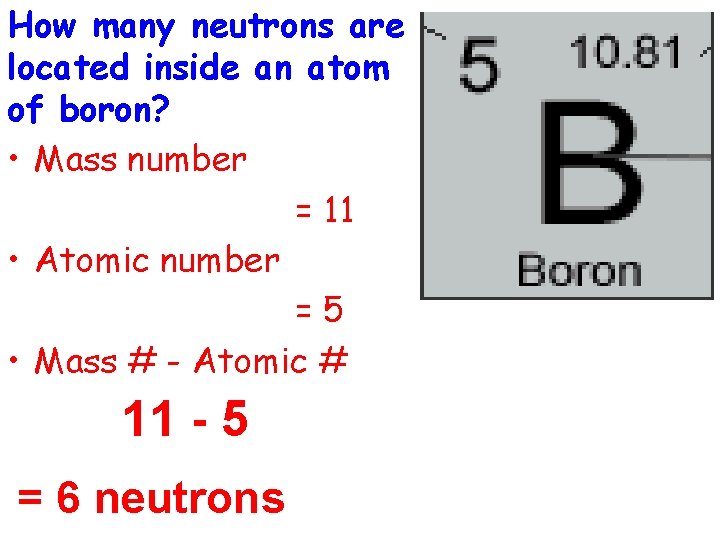
Atomic number: 5 Atomic mass: 10. 81 Mass Number: 11

How to determine the # of neutrons • Subtract mass number and atomic number Atomic number: 42 Mass Number: 96 # of neutrons: 96 – 42 = 54 neutrons

How many neutrons are located inside an atom of boron? • Mass number = 11 • Atomic number =5 • Mass # - Atomic # 11 - 5 = 6 neutrons

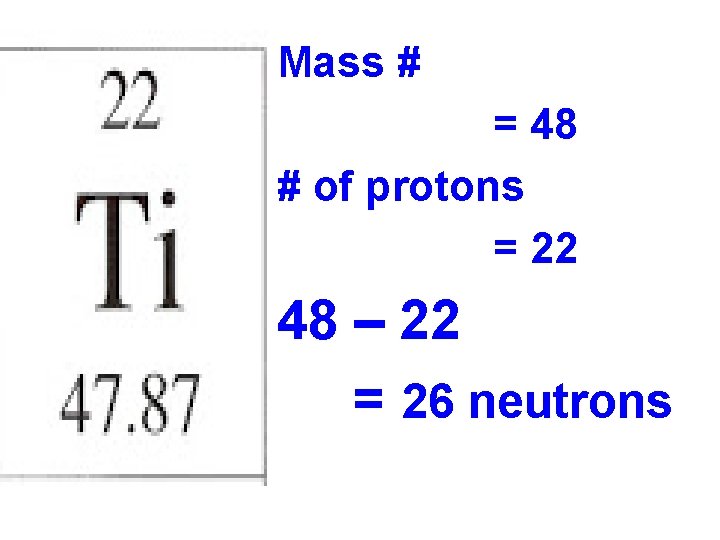
Mass # = 48 # of protons = 22 48 – 22 = 26 neutrons

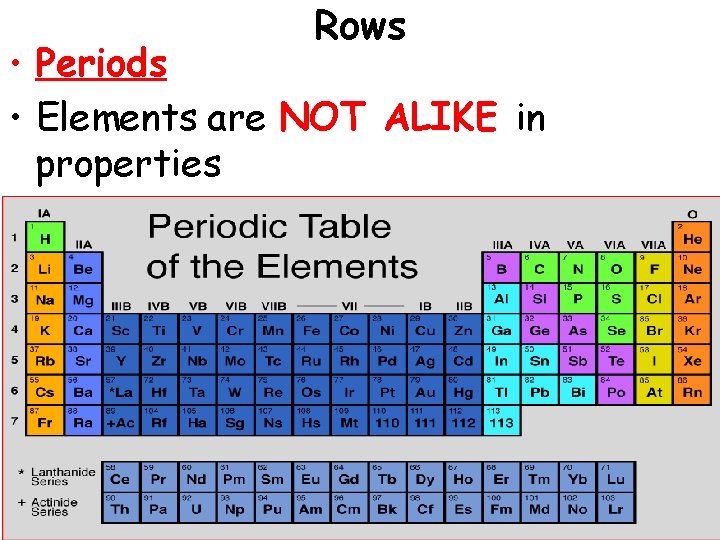
How are elements arranged? • By Atomic number • Increases as move from L R

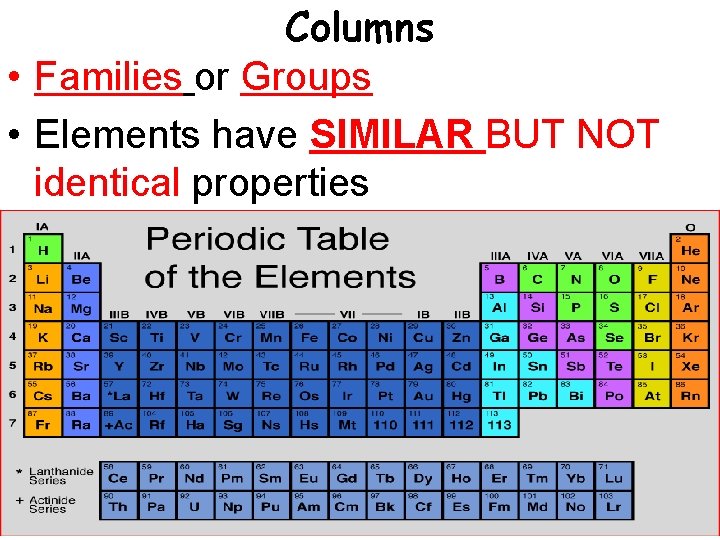
Columns • Families or Groups • Elements have SIMILAR BUT NOT identical properties

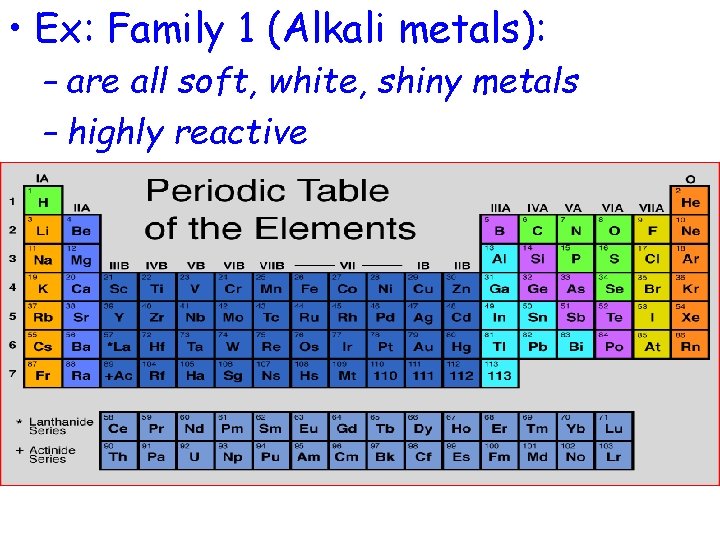
• Ex: Family 1 (Alkali metals): – are all soft, white, shiny metals – highly reactive

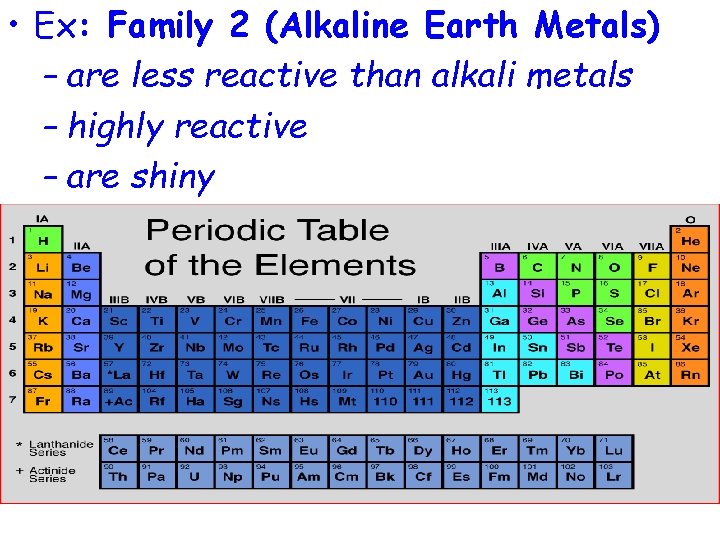
• Ex: Family 2 (Alkaline Earth Metals) – are less reactive than alkali metals – highly reactive – are shiny

Topic: Chemistry Aim: Explain how elements are classified in the Periodic Table of Elements. Do Now: Take out your Periodic Table notes. HW: Phases Reading Notes due Thursday.

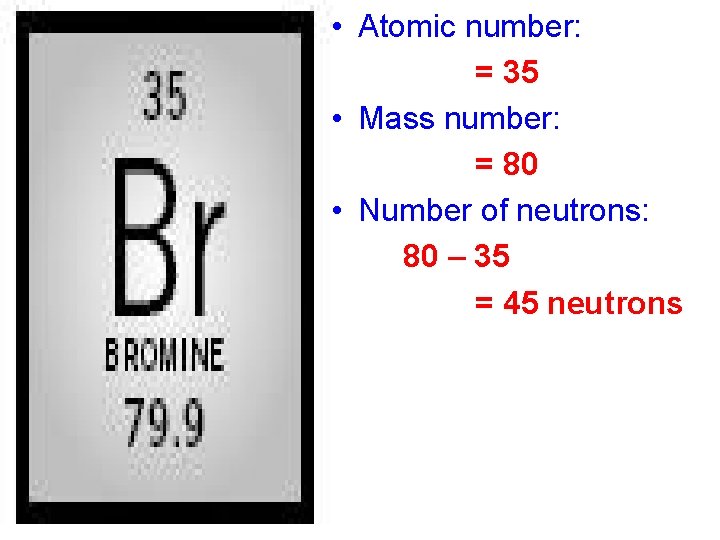
• Atomic number: = 35 • Mass number: = 80 • Number of neutrons: 80 – 35 = 45 neutrons

• How many electrons does Bromine have? When Br is neutral, it has the same number of protons and electrons. 35 electrons

42 protons 22 protons 5 protons 42 electrons 22 electrons 5 electrons

Rows • Periods • Elements are NOT ALIKE in properties

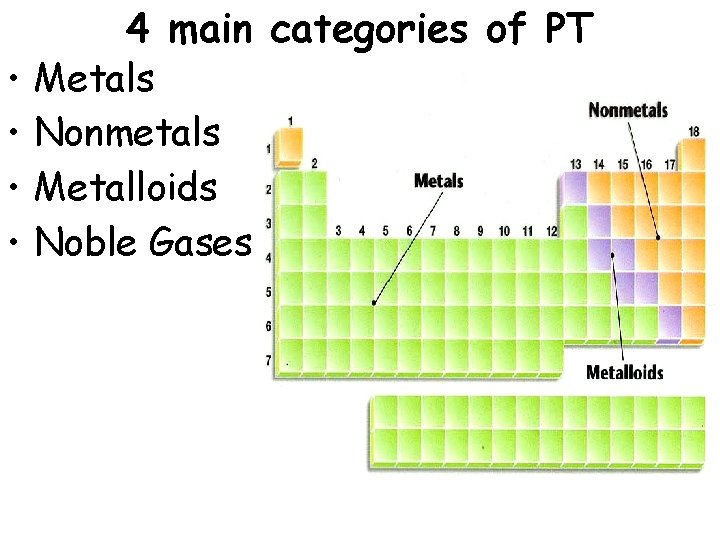
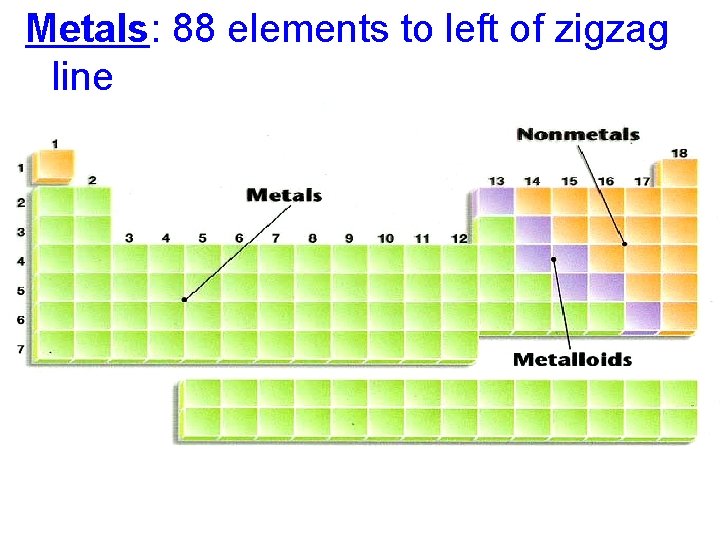
• • 4 main categories of PT Metals Nonmetals Metalloids Noble Gases

Metals: 88 elements to left of zigzag line

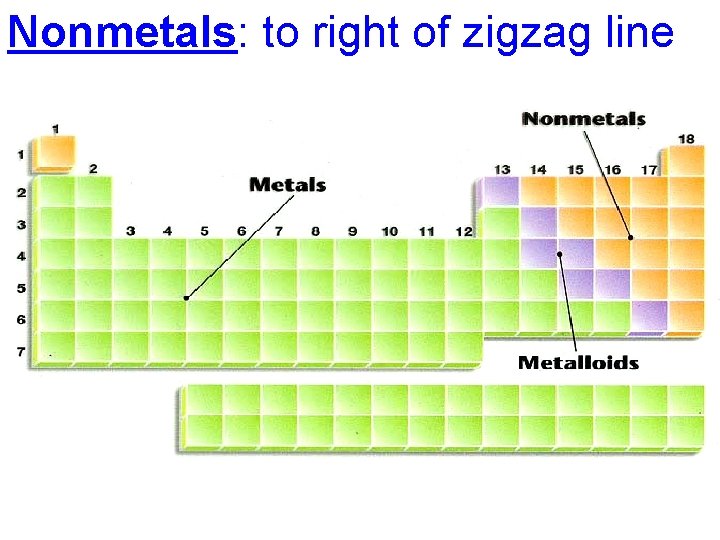
Nonmetals: to right of zigzag line

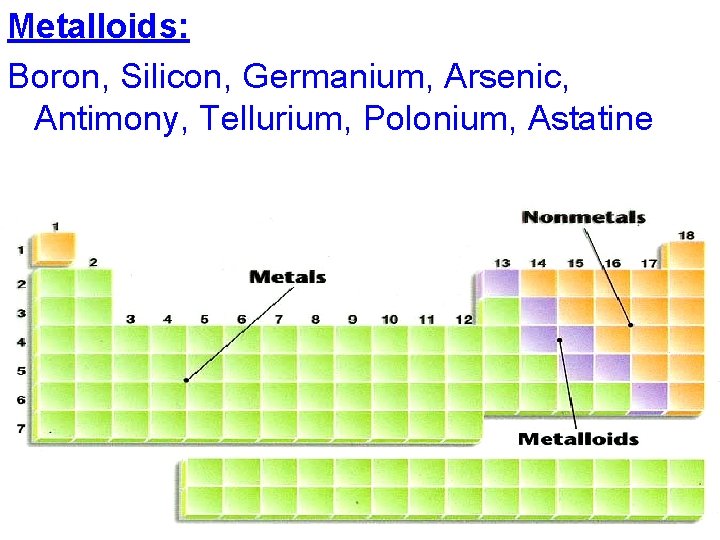
Metalloids: along both sides of the zigzag line

Metalloids: Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Polonium, Astatine

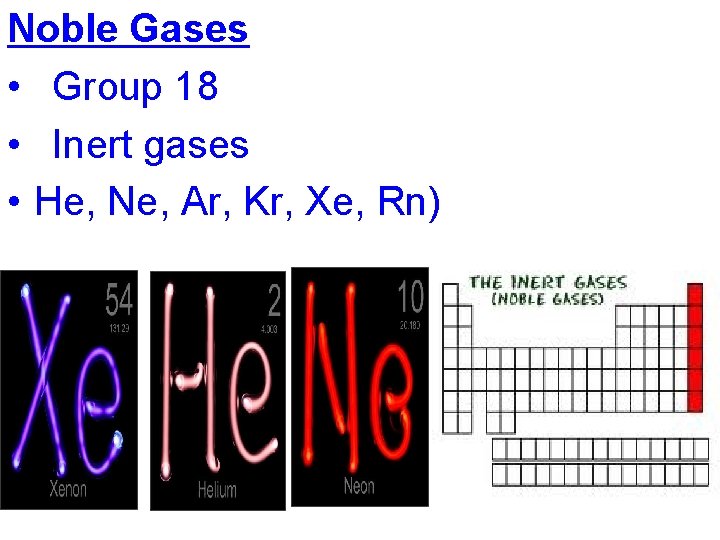
Noble Gases • Group 18 • Inert gases • He, Ne, Ar, Kr, Xe, Rn)

Noble gases glow brightly when an electric charge is passed through them, and so are used as advertising signs: neon tubes glow red, xenon blue, and krypton bluish-white; argon tubes glow pale red at low pressures, blue at high pressures.

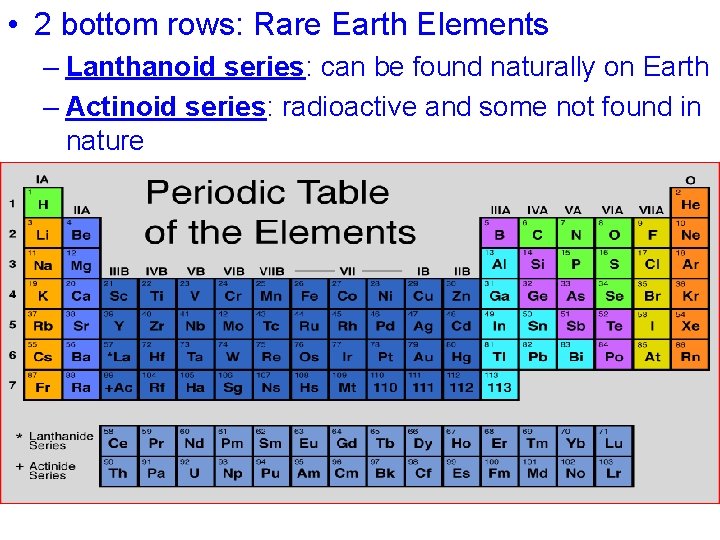
• 2 bottom rows: Rare Earth Elements – Lanthanoid series: can be found naturally on Earth – Actinoid series: radioactive and some not found in nature

Let’s summarize… 1. Explain how the periodic table of elements is arranged. 2. What does the decimal number in an element key represent? 3. How do you determine the mass number? 4. How can you determine the number of neutrons? 5. Identify the 4 categories of elements on the periodic table.

NUMBER OF NEUTRONS? Mass # = 112 # of protons = 48 112 – 48 = 64 neutrons

Review: 1. Elements in the periodic table arranged by 2. atomic number 3. atomic weight 4. number of neutrons 5. chemical reactivity

2. Which of these things will you NOT find in the periodic table? 1. Element symbol 2. Atomic weight 3. Atomic orbital radius 4. Atomic number

5. Which element is a metalloid? 1. S (Sulfur) 2. Si (Silicon) 3. Ba (Barium) 4. Br (Bromine)

Atomic number: 22 Atomic mass: 47. 87 Mass Number: 48



How many protons? 2 How many electrons? 2 How many neutrons? 4 -2 = 2

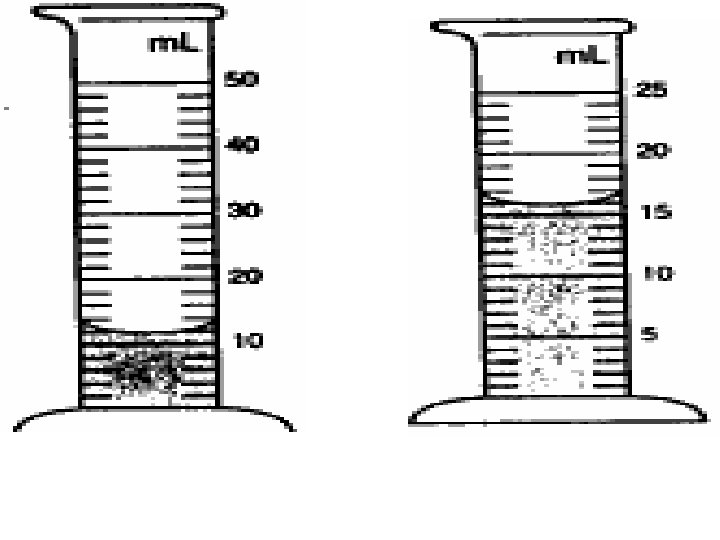
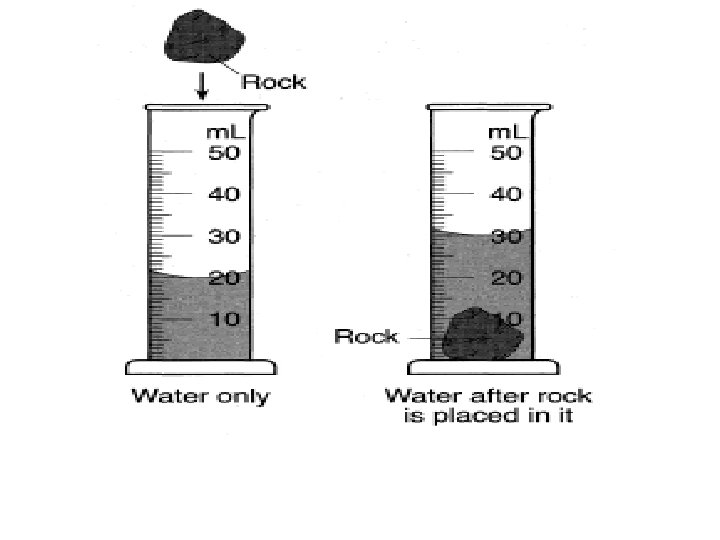
5. Volume is a. the amount of matter in an object b. the amount of space an object takes up. c. the gravitational pull on an object. d. an object's resistance to a change in motion.

3. A millimeter is equal to a. 0. 1 m b. 0. 01 m c. 0. 001 m d. 0. 0001 m

6. A liter of water contains a. 1000 m. L. b. 100 m. L. c. 10 m. L. d. 1 m. L




