AP CHEMISTRY CHAPTER 11 NOTES PROPERTIES OF SOLUTIONS







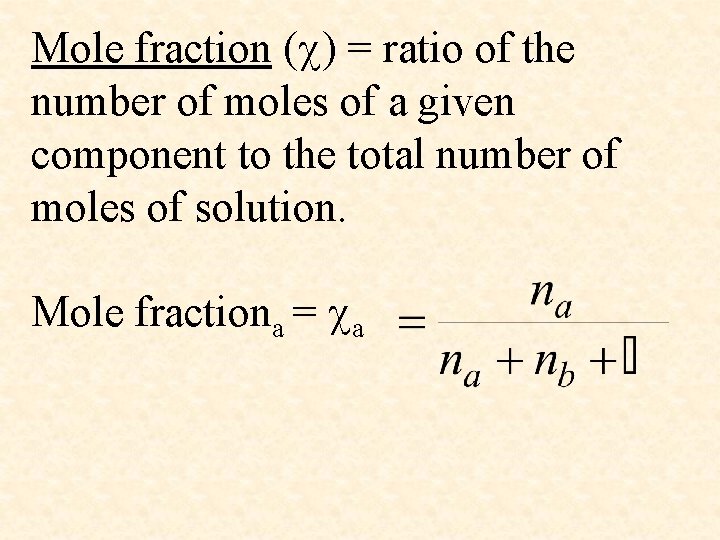










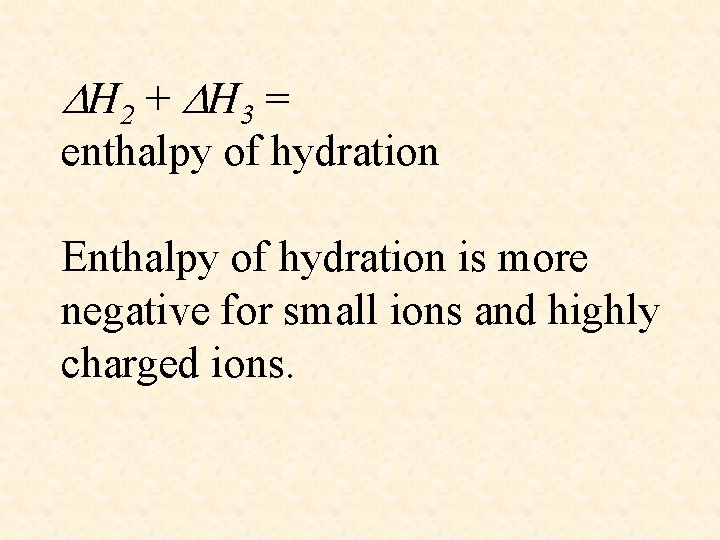












- Slides: 53

AP CHEMISTRY CHAPTER 11 NOTES PROPERTIES OF SOLUTIONS

Solution terms:

Solution- a homogeneous mixture (gases, liquids, or solids)

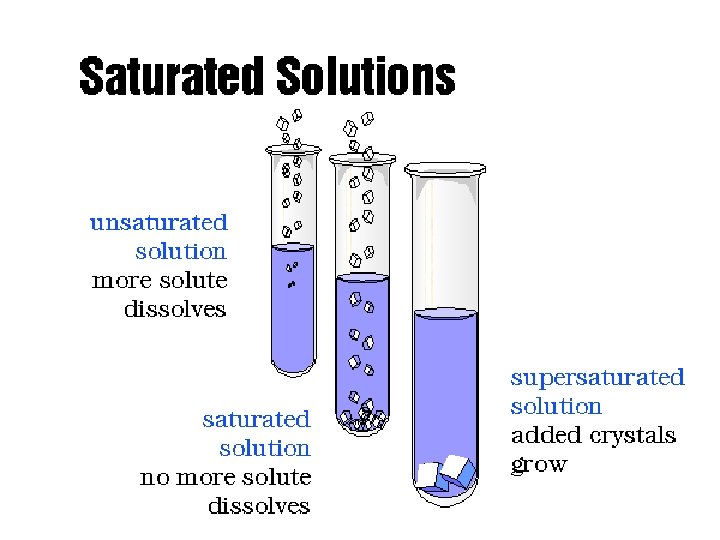
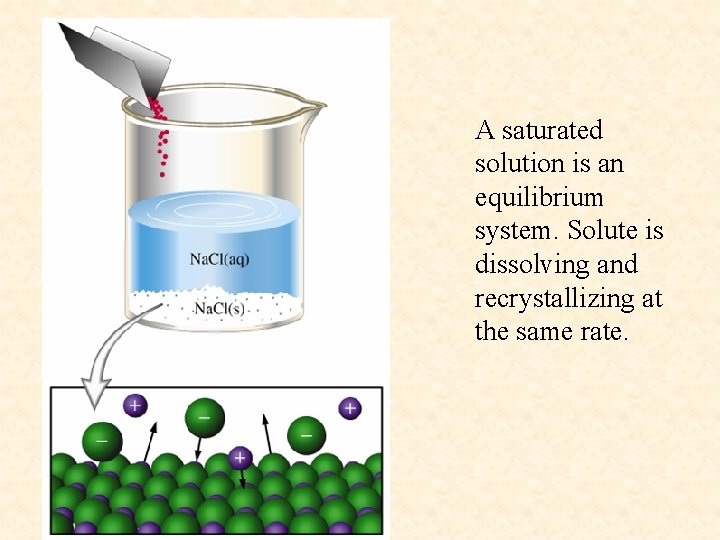
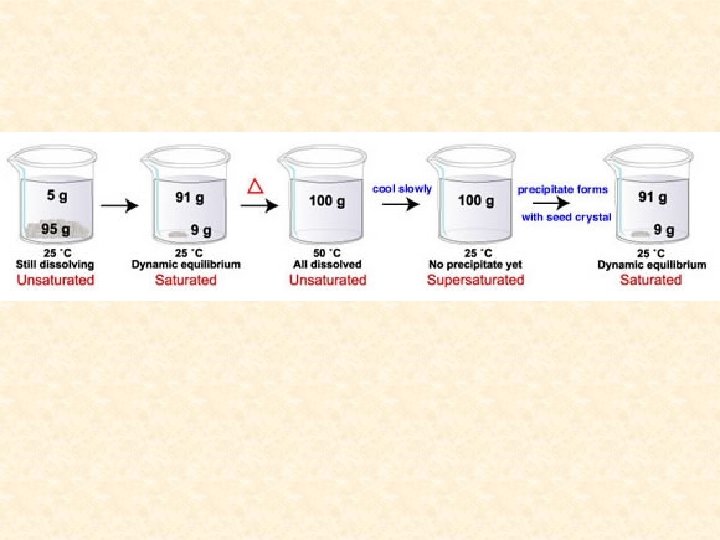
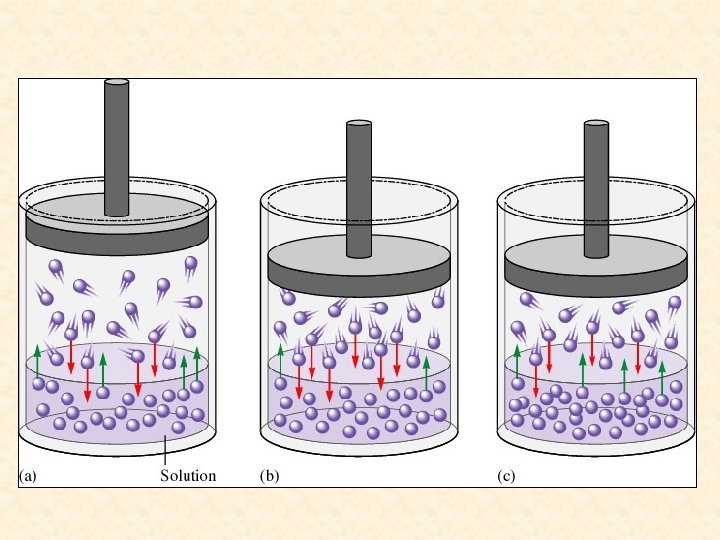
Saturated solution- a solution containing the maximum amount of solute that will dissolve under a given set of conditions. Saturated solutions are at dynamic equilibrium with any excess undissolved solute present. Solute particles dissolve and recrystallize at equal rates.

Unsaturated solution- a solution containing less than the maximum amount of solute that will dissolve under a given set of conditions. (more solute can dissolve)

Supersaturated solution- a solution that has been prepared at an elevated temperature and then slowly cooled. It contains more than the usual maximum amount of solution dissolved. A supersaturated solution is very unstable and the addition of a “seed crystal’; will cause all excess solute to crystallize out of solution leaving the remaining solvent saturated.


A saturated solution is an equilibrium system. Solute is dissolving and recrystallizing at the same rate.


Units of solution concentration:

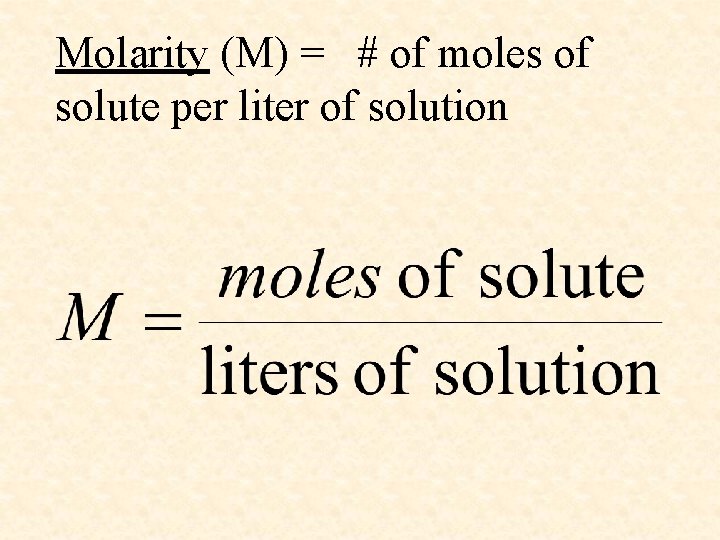
Molarity (M) = # of moles of solute per liter of solution
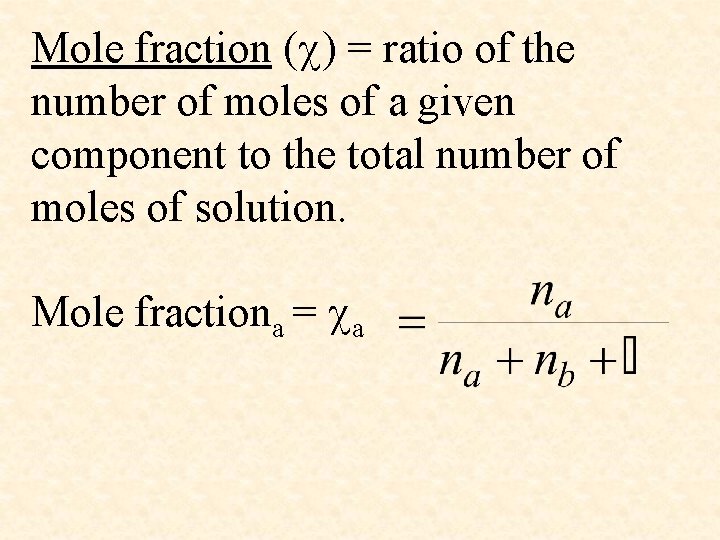
Mole fraction ( ) = ratio of the number of moles of a given component to the total number of moles of solution. Mole fractiona = a

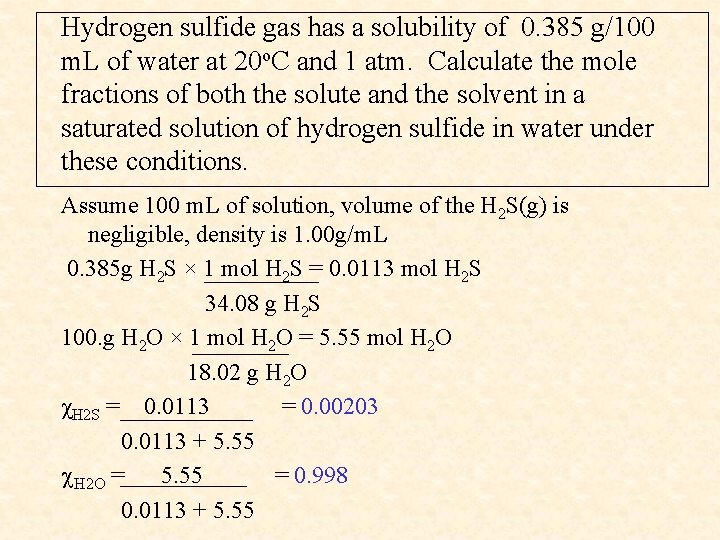
Hydrogen sulfide gas has a solubility of 0. 385 g/100 m. L of water at 20 o. C and 1 atm. Calculate the mole fractions of both the solute and the solvent in a saturated solution of hydrogen sulfide in water under these conditions. Assume 100 m. L of solution, volume of the H 2 S(g) is negligible, density is 1. 00 g/m. L 0. 385 g H 2 S × 1 mol H 2 S = 0. 0113 mol H 2 S 34. 08 g H 2 S 100. g H 2 O × 1 mol H 2 O = 5. 55 mol H 2 O 18. 02 g H 2 O χH 2 S = 0. 0113 = 0. 00203 0. 0113 + 5. 55 H 2 O = 5. 55 = 0. 998 0. 0113 + 5. 55

Energies involved in solution formation:

Whether or not a solute dissolves in a solvent depends upon the strengths of 3 different types of attractions, as well as a change in the entropy (disorder) of the system. 1. Attraction of solute particles for each other 2. Attraction of solvent particles for each other 3. Attraction of solute particles for solvent particles These 3 attractions add up to give the overall heat of solution.


“like dissolves like” -general observation for solubility, not a reason Substances with similar types of intermolecular forces dissolve in each other. Polar solvents dissolve polar or ionic solutes. Nonpolar solvents dissolve nonpolar solutes.

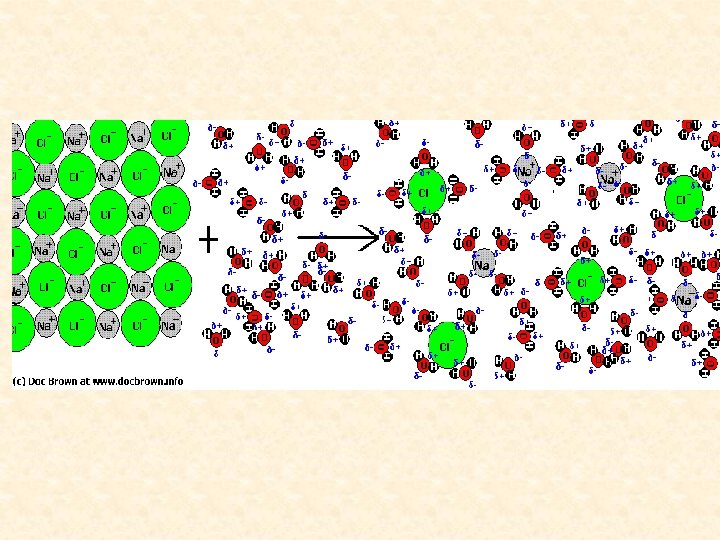
Water dissolves many salts because the strong ion-dipole attractions that water forms with the ions are very similar to the strong attractions between the ions themselves.

The same salts are insoluble in hexane (C 6 H 14) because the weak LDF forces their ions could form with this nonpolar solvent are much weaker than the attraction between ions.

Oil does not dissolve in water because the LDF-dipole forces are much weaker than the hydrogen bonding of water.

Solubilities of alcohols in water: As the hydrocarbon portion of the alcohol increases in length, the alcohol becomes less soluble. (More of the molecule is nonpolar. ) The opposite situation would exist if hexane were the solvent.

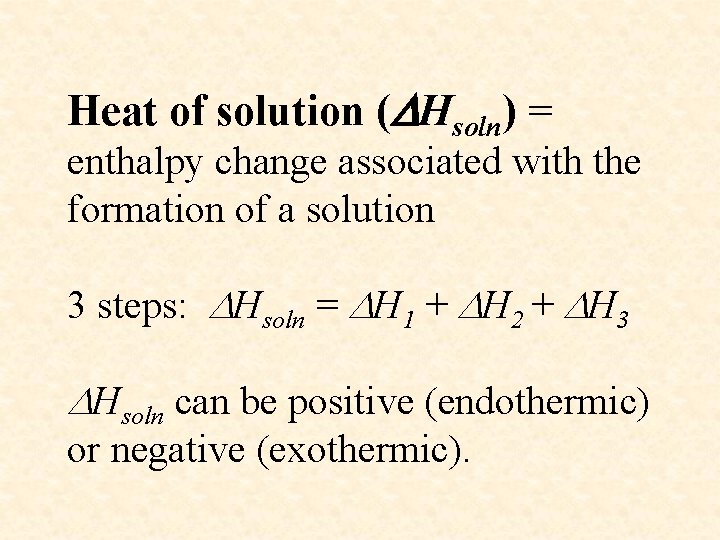
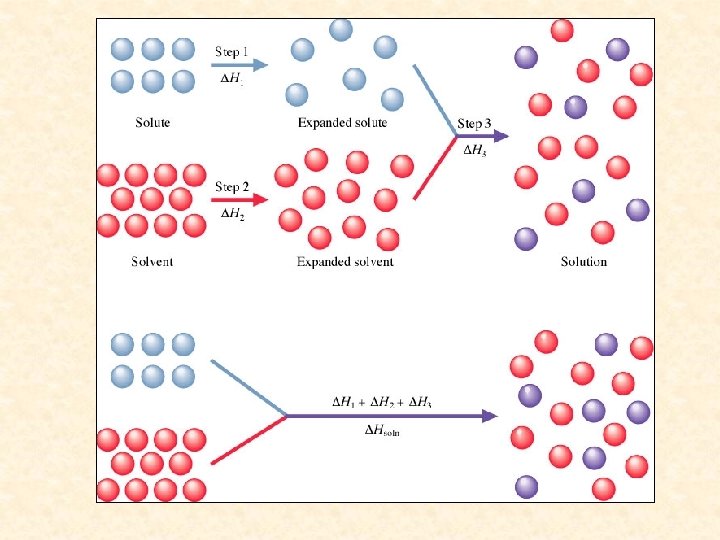
Heat of solution ( Hsoln) = enthalpy change associated with the formation of a solution 3 steps: Hsoln = H 1 + H 2 + H 3 Hsoln can be positive (endothermic) or negative (exothermic).

Step 1 = Breaking up solute (endothermic) “expanding the solute” High in ionic and polar solutes, low in nonpolar solutes Hsolute = − Hlattice energy

Step 2 = Breaking up solvent (endothermic) “expanding the solvent” High in polar solvent, low in nonpolar solvent

Step 3 = Interaction of solute and solvent (exothermic) (High negative in polar-polar, low negative in rest)
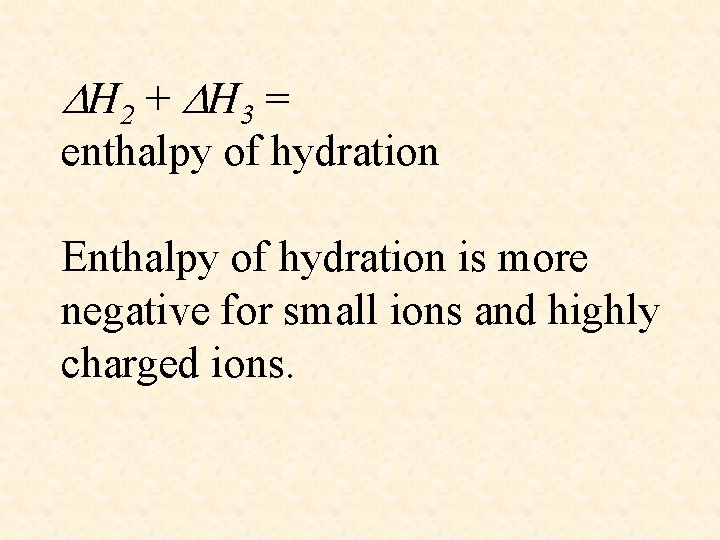
H 2 + H 3 = enthalpy of hydration Enthalpy of hydration is more negative for small ions and highly charged ions.


Most heats of solution are positive (endothermic). The reason that the solute dissolves is that the solution process usually increases the entropy (disorder). This makes the process thermodynamically favorable. The solution process involves two factors, the change in heat and the change in entropy, and their relative sizes determine whether a solute dissolves in a solvent.

Factors Affecting Solubility:

Molecular Structure: Fat soluble vitamins, (A, D, E, K) –nonpolar (can be stored in the body tissue) Water soluble vitamins, (B&C) –polar (are not stored, must be consumed regularly) Hydrophobic- water fearing (nonpolar) Hydrophilic – water loving (polar)

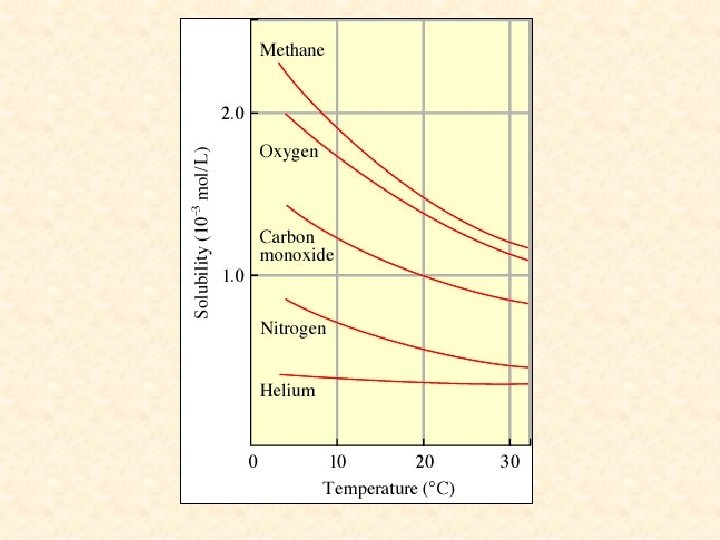
Pressure Effects: The solubility of a gas is higher with increased pressure. Pressure had very little affect on the solubility of liquids and solids

Henry’s Law- the amount of a gas dissolved in a solution is directly proportional to the pressure of the gas above the solution.


Henry’s Law is obeyed best for dilute solutions of gases that don’t dissociate or react with the solvent.

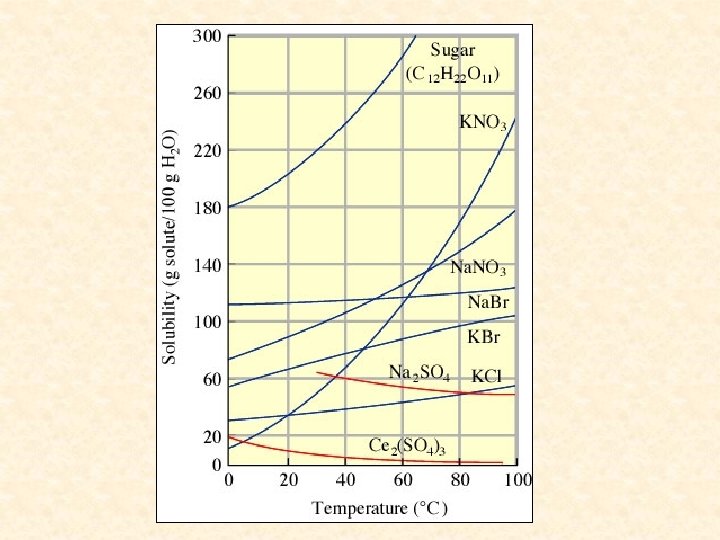
Temperature Effects: The amount of solute that will dissolve usually increases with increasing temperature but may decrease. Solubility generally increases with temperature if the solution process is endothermic ( Hsoln > 0 ). Solubility generally decreases with temperature if the solution process is exothermic ( Hsoln < 0).


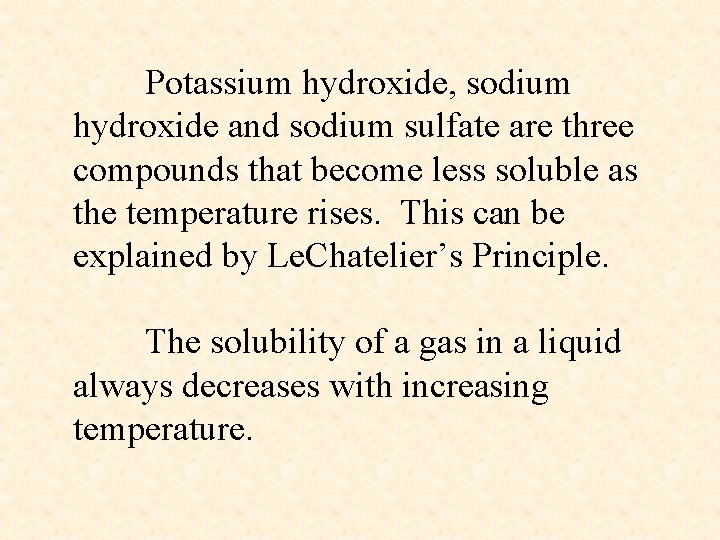
Potassium hydroxide, sodium hydroxide and sodium sulfate are three compounds that become less soluble as the temperature rises. This can be explained by Le. Chatelier’s Principle. The solubility of a gas in a liquid always decreases with increasing temperature.


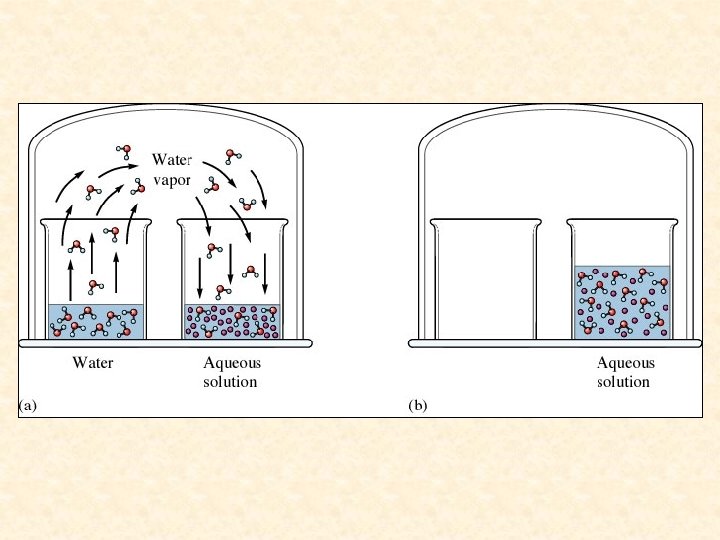
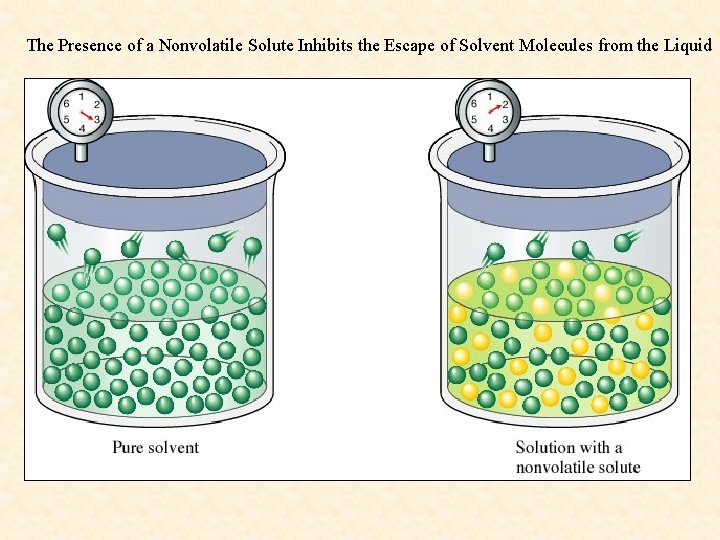
Vapor Pressure Lowering- While the presence of a volatile solute (more volatile than the solvent) increases the vapor pressure of a solution, the presence of a nonvolatile solute lowers the vapor pressure of a solvent. This is because the dissolved nonvolatile solute decreases the number of solvent molecules per unit volume. (Nonvolatile solute dilutes the solution). There are fewer solvent molecules on the surface to escape.


The Presence of a Nonvolatile Solute Inhibits the Escape of Solvent Molecules from the Liquid

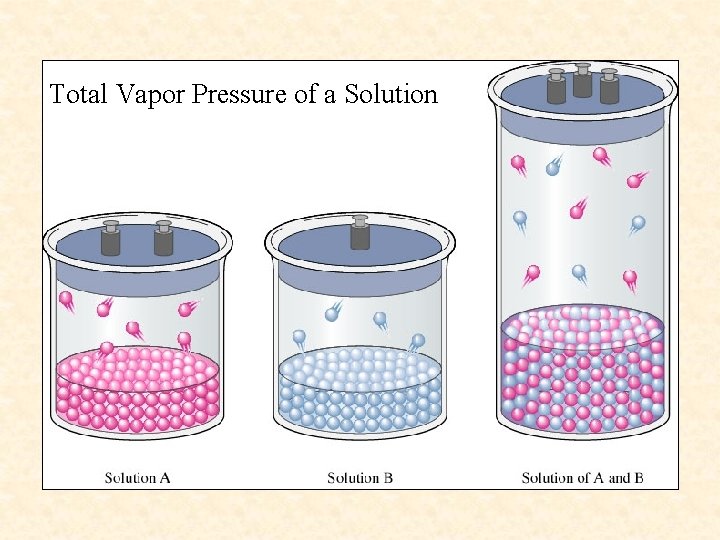
Liquid-Liquid solutions- Solutions in which both solute and solvent are liquid and the liquids are volatile do not behave ideally. Both solute and solvent contribute to the vapor pressure. If the solute is more volatile than the solvent, the vapor pressure of the solution is higher than the vapor pressure of the solvent.

Total Vapor Pressure of a Solution

Colloids- (also called colloidal dispersions) - a suspension of tiny particles in some medium. The dispersed colloidal particles are larger than a simple molecule but small enough to remain distributed and not settle out. A colloidal particle has a diameter between 1 and 1000 nm and may contain many atoms, ions, or molecules. Because of their small particle size, colloids have an enormous total surface area.

The particles stay suspended because of electrostatic repulsion. Coagulation occurs by heating (particles collide so hard that they stick together) or by the addition of an electrolyte (neutralizes ion layers). The particles can be separated by filtration. Foams, aerosols, emulsions, and sols are various types of colloidal dispersions.

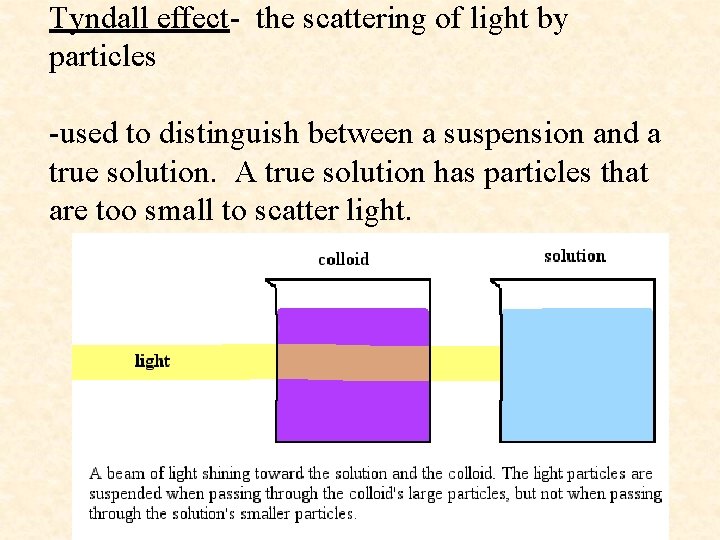
Tyndall effect- the scattering of light by particles -used to distinguish between a suspension and a true solution. A true solution has particles that are too small to scatter light.

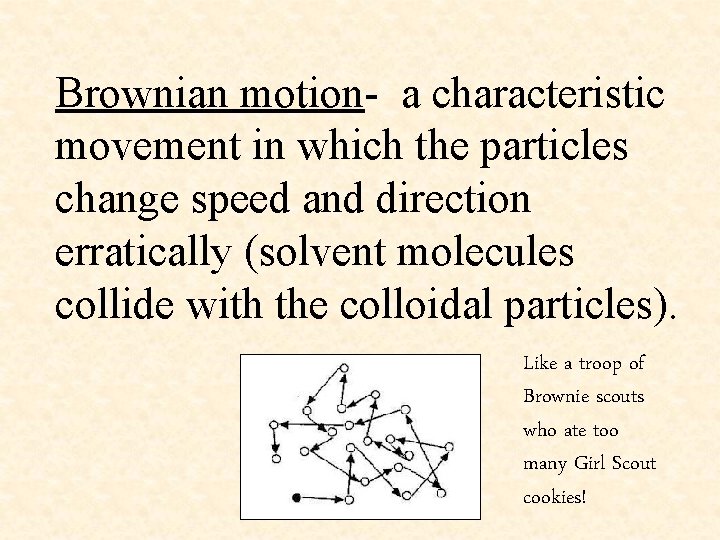
Brownian motion- a characteristic movement in which the particles change speed and direction erratically (solvent molecules collide with the colloidal particles). Like a troop of Brownie scouts who ate too many Girl Scout cookies!

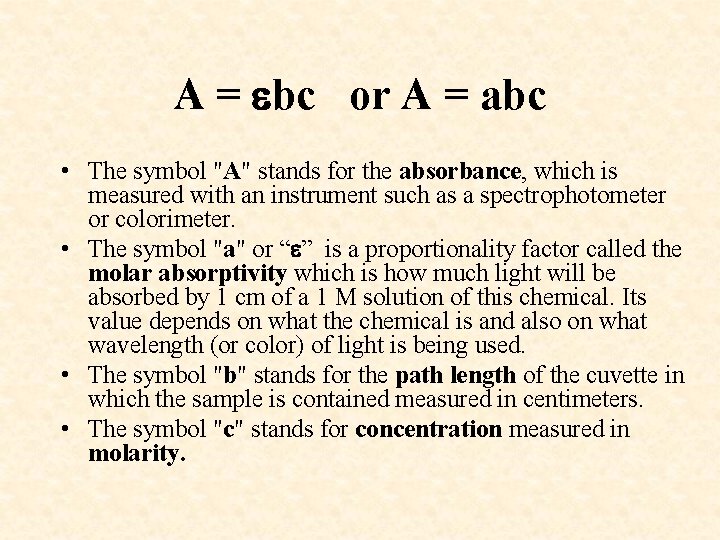
BEER’S LAW A = bc or A = abc Solution concentration can sometimes be measured by analyzing the intensity of the color of a solution through the use of a colorimeter or spectrophotometer. Beer's Law is a way of summarizing and quantifying the relationship between the absorbance of light of a given wavelength, the nature of the absorbing chemical, the path length of the solution, and the concentration of the solution. It expresses the ideal situation in which these factors are truly proportional to the absorbance.

A = bc or A = abc • The symbol "A" stands for the absorbance, which is measured with an instrument such as a spectrophotometer or colorimeter. • The symbol "a" or “ ” is a proportionality factor called the molar absorptivity which is how much light will be absorbed by 1 cm of a 1 M solution of this chemical. Its value depends on what the chemical is and also on what wavelength (or color) of light is being used. • The symbol "b" stands for the path length of the cuvette in which the sample is contained measured in centimeters. • The symbol "c" stands for concentration measured in molarity.

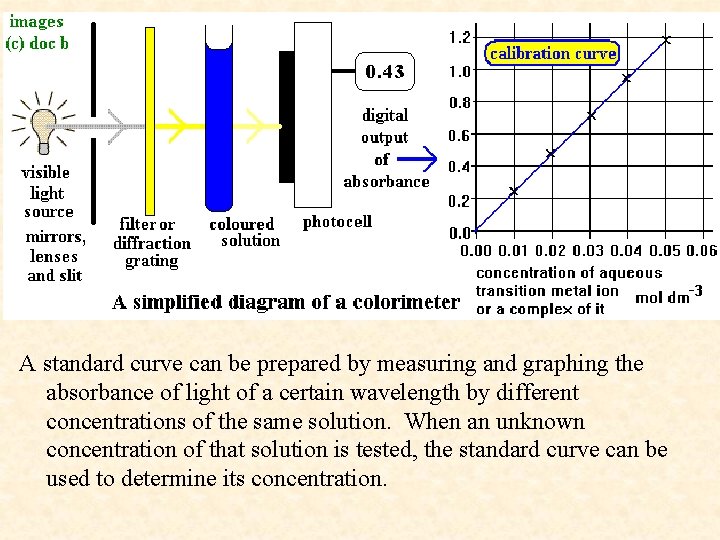
A standard curve can be prepared by measuring and graphing the absorbance of light of a certain wavelength by different concentrations of the same solution. When an unknown concentration of that solution is tested, the standard curve can be used to determine its concentration.


2012
