Electron Configurations Ms Hughes Modern Chemistry Chapter 4












- Slides: 24

Electron Configurations Ms. Hughes Modern Chemistry Chapter 4

• The quantum model of the atom improves on the Bohr model because it describes the arrangements of electrons in atoms other than hydrogen. • The arrangement in an atom is known as the atom’s electron configuration. • All atoms have different numbers of electrons so they all have unique configurations. • Electrons in atoms tend to assume the arrangements that have the lowest possible energies. The lowest energy arrangement of the electrons for each element is called the groundstate configuration.

Rules Governing Electron Configurations • First the energy levels of the orbitals are determined. Then electrons are added to the orbitals, one by one, according to three basic rules. • According to the Aufbau principle, an electron occupies the lowest energy orbital that can receive it. • The orbital with the lowest energy is the 1 s orbital.


• 1 s first • 2 s second • 2 p third • Beginning with the 3 rd main energy level (n=3), the energies of sublevels of different main energy levels begin to overlap


• 4 s sublevel is lower than 3 d in energy. • Therefore we add to the 4 s then come back to the 3 d until it is filled and missing only 2 electrons. At this point we take the two from the 4 s and refill 4 s when we move back up. • Website example atoms

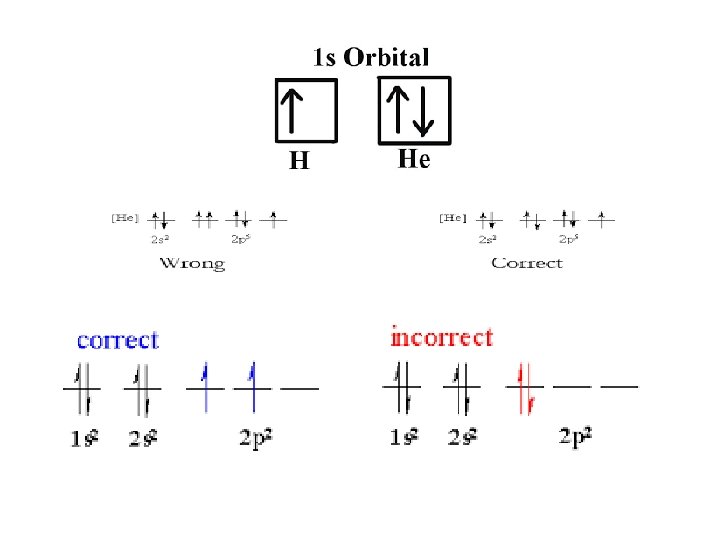
• Pauli’s exclusion principle – no two electrons in the same atom can have the same set of four quantum numbers. The two values of spin quantum number reflect the fact that for two electrons to occupy the same orbital they must have opposite spin states. • In this way electron-electron repulsion is minimized so that the electron arrangements have the lowest energy possible.

• Hund’s Rule – orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons of a singly occupied orbital must have the same spin state. • You must fill each level with 1 (all having the same spin) before going back to add a 2 nd


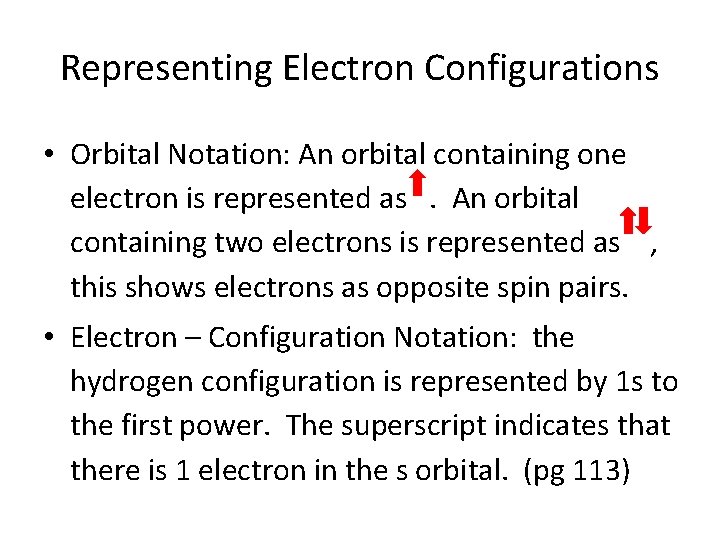
Representing Electron Configurations • Orbital Notation: An orbital containing one electron is represented as. An orbital containing two electrons is represented as , this shows electrons as opposite spin pairs. • Electron – Configuration Notation: the hydrogen configuration is represented by 1 s to the first power. The superscript indicates that there is 1 electron in the s orbital. (pg 113)

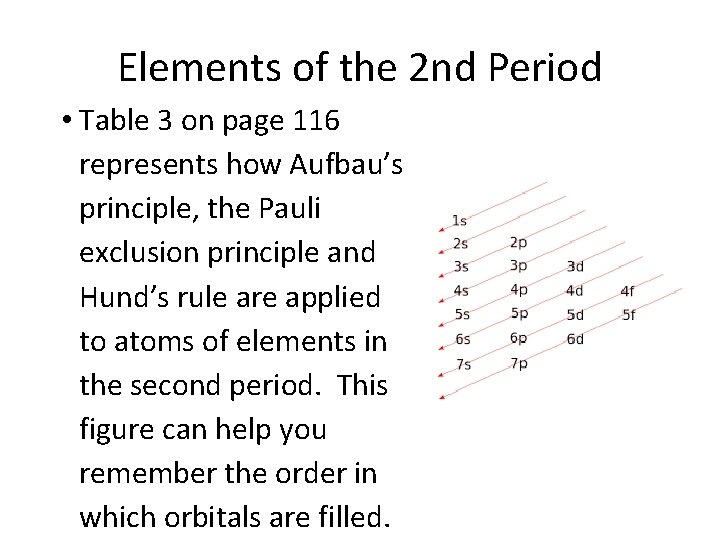
Elements of the 2 nd Period • Table 3 on page 116 represents how Aufbau’s principle, the Pauli exclusion principle and Hund’s rule are applied to atoms of elements in the second period. This figure can help you remember the order in which orbitals are filled.

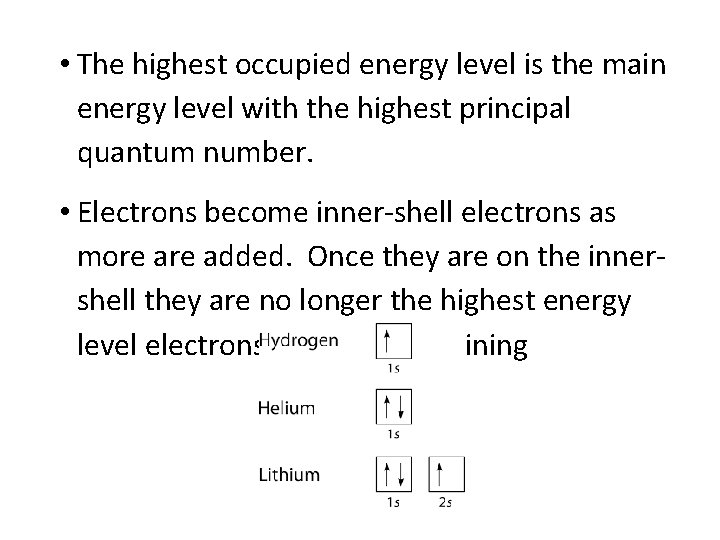
• The highest occupied energy level is the main energy level with the highest principal quantum number. • Electrons become inner-shell electrons as more added. Once they are on the innershell they are no longer the highest energy level electrons. electron-containing

Elements of the Third Period • After the outer octet (8)is filled in neon, the next electron enters the s sublevel in the n=3 main energy level. • the first 10 electrons in an atom of each of the third-period elements have the same configuration as neon. • This allows us to use shorthand notation for the electron configurations of third-period

Noble-Gas Notation • Group 18 elements are called the noble gases. to simplify sodium’s notation the symbol for neon enclosed in square brackets [Ne] is used to represent the complete neon configuration. • [Ne] = 1 s 22 p 6 • [Ne]3 s 1 - Name this element • Noble gas configuration refers to an outer main energy level occupied, in most cases by 8 electrons.

Fourth Period Elements • The first element in the 4 th period is K, which has the electron configuration [Ar]4 s 1. • The next element is Ca = [Ar]4 s 2. • Now complete the 3 rd element in the 4 th period.

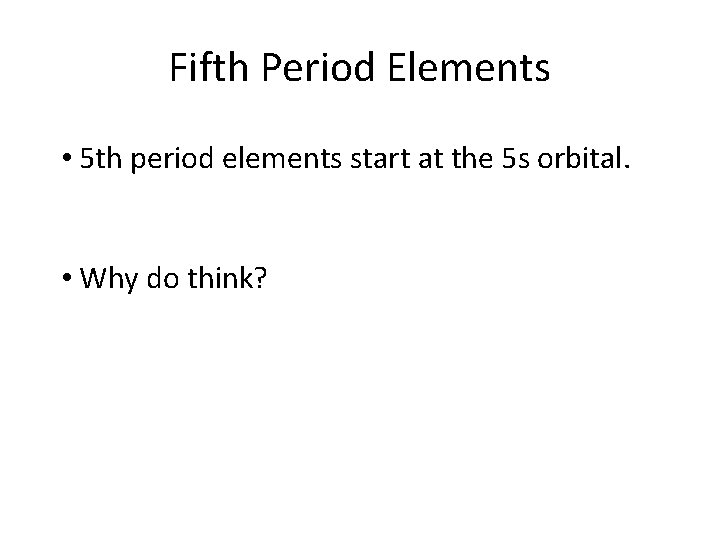
Fifth Period Elements • 5 th period elements start at the 5 s orbital. • Why do think?

Complete Sample Problem B on page 120 1. Write bothe complete electronconfiguration notation and the noble gas notation for Fe. 2. How many electron-containing orbitals are in an atom of iron? How many of these orbitals are completely filled? How many unpaired electrons are there in an atom of Fe? In which sublevel are the unpaired electrons?

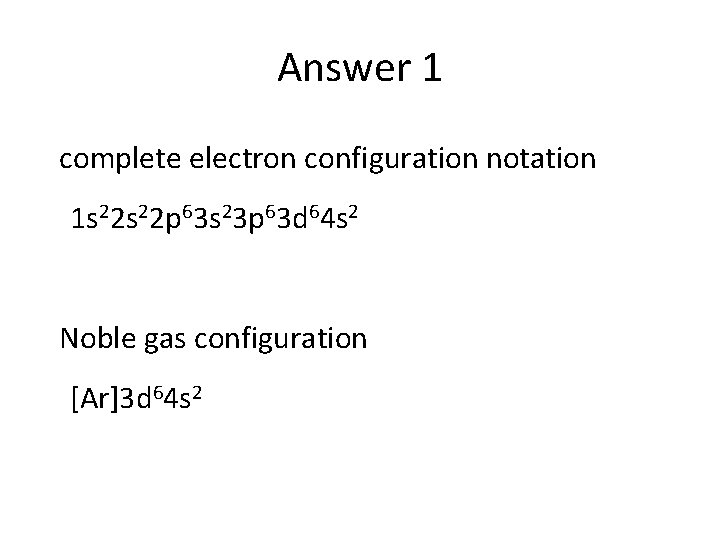
Answer 1 complete electron configuration notation 1 s 22 p 63 s 23 p 63 d 64 s 2 Noble gas configuration [Ar]3 d 64 s 2

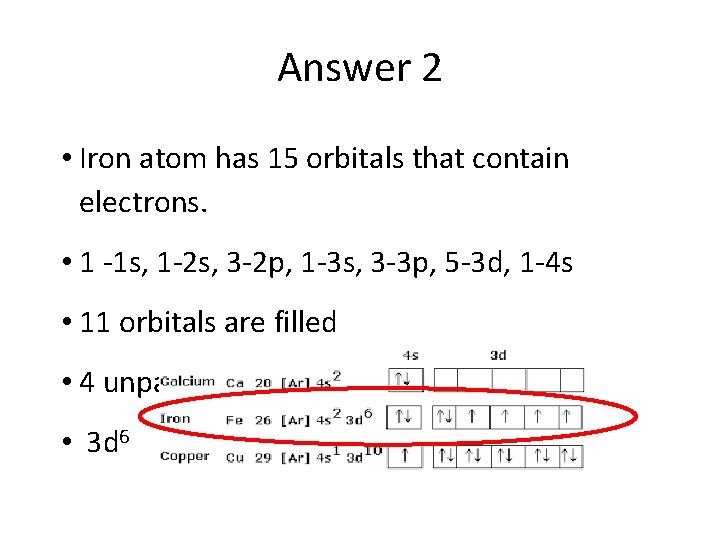
Answer 2 • Iron atom has 15 orbitals that contain electrons. • 1 -1 s, 1 -2 s, 3 -2 p, 1 -3 s, 3 -3 p, 5 -3 d, 1 -4 s • 11 orbitals are filled • 4 unpaired electrons in the 3 d sublevel • 3 d 6

Practice problems pg 121 1 -4 all

Sixth & Seventh Period Elements Ce, cerium, the 4 f orbitals begin to fill, giving cerium atoms a configuration of [Xe]4 f 15 d 16 s 2

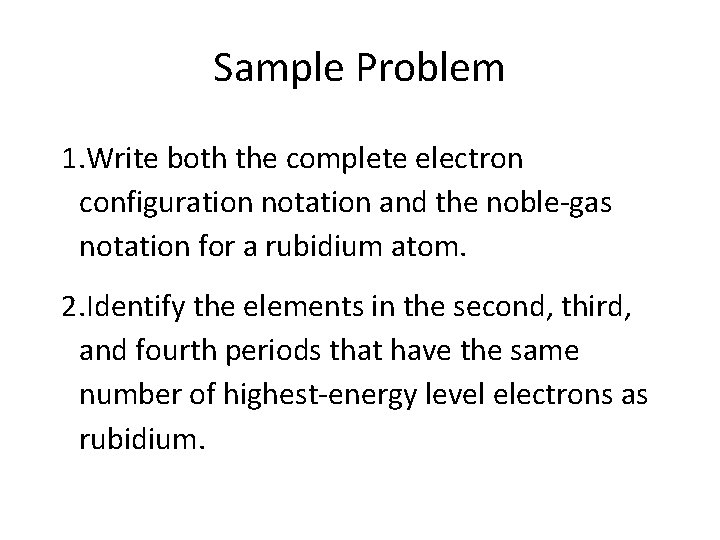
Sample Problem 1. Write both the complete electron configuration notation and the noble-gas notation for a rubidium atom. 2. Identify the elements in the second, third, and fourth periods that have the same number of highest-energy level electrons as rubidium.

Solution 1 1. 1 s 22 p 63 s 23 p 63 d 104 s 24 p 65 s 1 [Kr]5 s 1 2. 1 electron in it’s highest energy level (5 s) elements with same outermost configuration are in the 2 nd period, Li; 3 rd period Na; 4 period k