CHAPTER 2 CHEMISTRY COMES ALIVE Section 2 c


















- Slides: 22

CHAPTER 2 CHEMISTRY COMES ALIVE Section 2 c

Biochemistry The study of the chemical composition and reactions of living matter.

Biochemistry • Organic compounds – Contain carbon, are covalently bonded, and are often large • Inorganic compounds – All other chemicals in the body – Do not contain carbon – Water, salts, and many acids and bases

Today • We will go over – Water – Salts – Acids – Bases – p. H – Buffers

Water Most abundant and important inorganic compound in living material. Makes up 60% - 80% of the volume of most living cells.

Properties of Water • High heat capacity – absorbs and releases large amounts of heat before changing temperature – This prevents sudden changes in body temperature cased by external factors like sun or wind or internal factors like heat released during vigorous muscle activity – As a part of blood, water redistributes heat among body tissues, ensuring temperature homeostasis

Properties of Water • High heat of vaporization – changing from a liquid to a gas requires large amounts of heat – As we sweat, perspiration (mostly water) evaporates from our skin removing large amounts of heat – This is a very efficient cooling mechanism for our bodies!

Properties of Water • Polar solvent properties – Water is often called the universal solvent – dissolves ionic substances – forms hydration layers around large charged molecules – serves as the body’s major transport medium

Properties of Water • Reactivity – an important part of hydrolysis and dehydration synthesis reactions – Food is digested to their building blocks by adding a water molecule to each bond - Hydrolysis reactions – Carbohydrates and proteins are synthesized from smaller molecules by removing a water molecule for each bond formed – dehydration synthesis

Properties of Water • Cushioning – resilient cushion around certain body organs – Helps protect organs from physical trauma – Cerebrospinal fluid surrounding the brain is an example

Salts

Salts • Inorganic compounds • Contain cations other than H+ and anions other than OH– • Are electrolytes; they conduct electrical currents

Salts • Maintaining proper ionic balance in our body fluids is one the most crucial homeostatic roles of the kidneys. • When this balance is severely disturbed, virtually nothing in the body works.

Acids and Bases are also electrolytes, Conduct electrical current

Acids • Tastes sour • Dissolves many metals • Acids release H+ and are therefore proton donors HCl H+ + Cl – Acids are proton donors!

Bases • Tastes bitter • Feel slippery • Bases release OH– and are proton acceptors Na. OH Na+ + OH– Bases are proton acceptors!

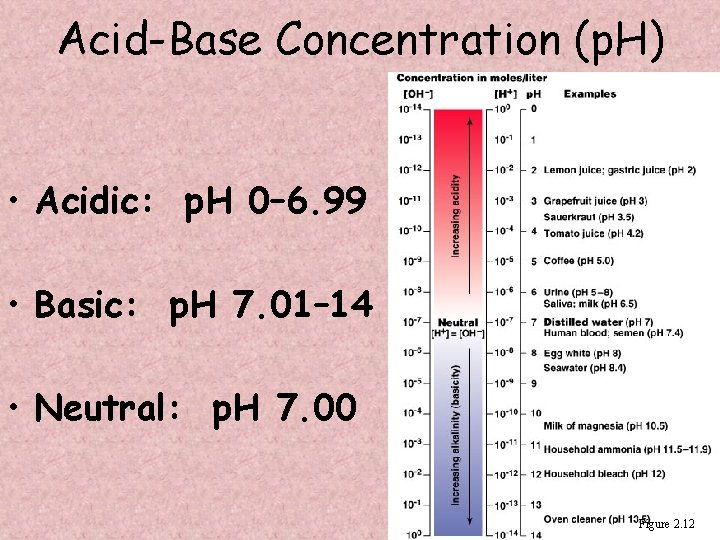
Acid-Base Concentration (p. H) • Acidic solutions have higher H+ concentration and therefore a lower p. H • Alkaline (basic) solutions have lower H+ concentration and therefore a higher p. H • Neutral solutions have equal H+ and OH– concentrations

Acid-Base Concentration (p. H) • Acidic: p. H 0– 6. 99 • Basic: p. H 7. 01– 14 • Neutral: p. H 7. 00 Figure 2. 12

Neutralization • When acids and bases are mixed, they react with each other in a displacement reaction to form water and a salt. HCl + Na. OH -> Na. Cl + H 2 O

Buffers • Systems that resist abrupt and large swings in the p. H of body fluids are buffering systems • If blood p. H varies from the narrow range of 7. 35 – 7. 45 by more than a few tenths, it can be fatal! • Homeostasis of acid-base balance is regulated by the kidneys and lungs and by chemical systems called Buffers.

Buffer systems • Carbonic acid-bicarbonate system is a very important chemical blood buffer – Carbonic acid dissociates reversibly, releasing bicarbonate ions and protons – The chemical equilibrium between carbonic acid and bicarbonate resists p. H changes in the blood

Quiz Next time!