Chapter 1 Density SI derived unit for density














![Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Na+ Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Na+](https://slidetodoc.com/presentation_image_h2/6632e5656b2d9eaebbc77ba14d846ad9/image-55.jpg)








- Slides: 88

Chapter 1

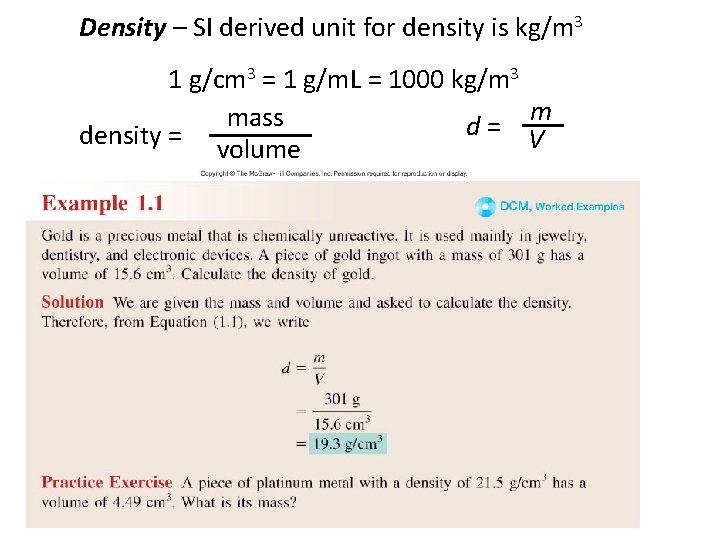
Density – SI derived unit for density is kg/m 3 1 g/cm 3 = 1 g/m. L = 1000 kg/m 3 m mass d= V density = volume

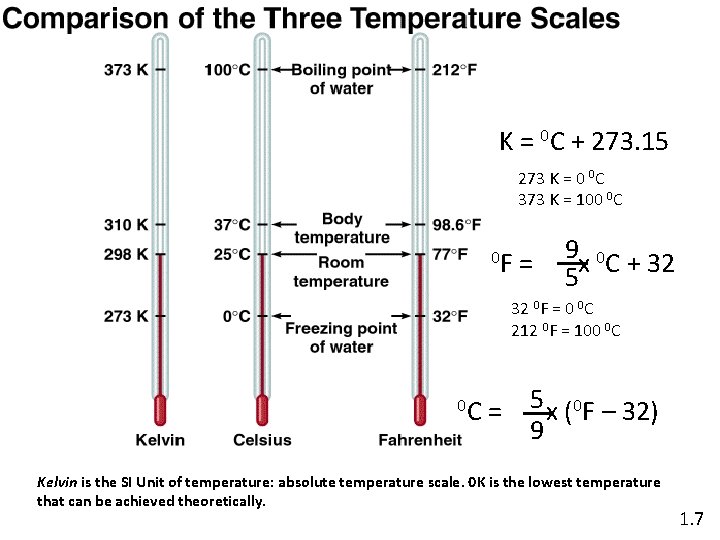
K = 0 C + 273. 15 273 K = 0 0 C 373 K = 100 0 C 0 F = 9 x 0 C + 32 5 32 0 F = 0 0 C 212 0 F = 100 0 C 0 C = 5 x (0 F – 32) 9 Kelvin is the SI Unit of temperature: absolute temperature scale. 0 K is the lowest temperature that can be achieved theoretically. 1. 7

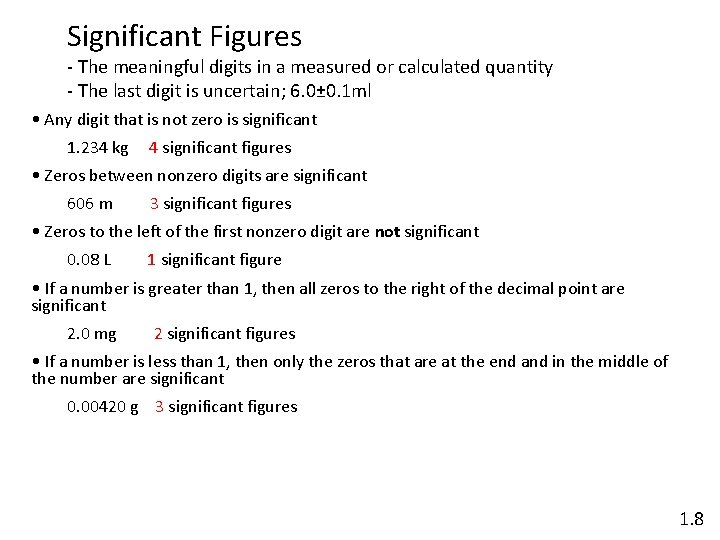
Significant Figures - The meaningful digits in a measured or calculated quantity - The last digit is uncertain; 6. 0± 0. 1 ml • Any digit that is not zero is significant 1. 234 kg 4 significant figures • Zeros between nonzero digits are significant 606 m 3 significant figures • Zeros to the left of the first nonzero digit are not significant 0. 08 L 1 significant figure • If a number is greater than 1, then all zeros to the right of the decimal point are significant 2. 0 mg 2 significant figures • If a number is less than 1, then only the zeros that are at the end and in the middle of the number are significant 0. 00420 g 3 significant figures 1. 8

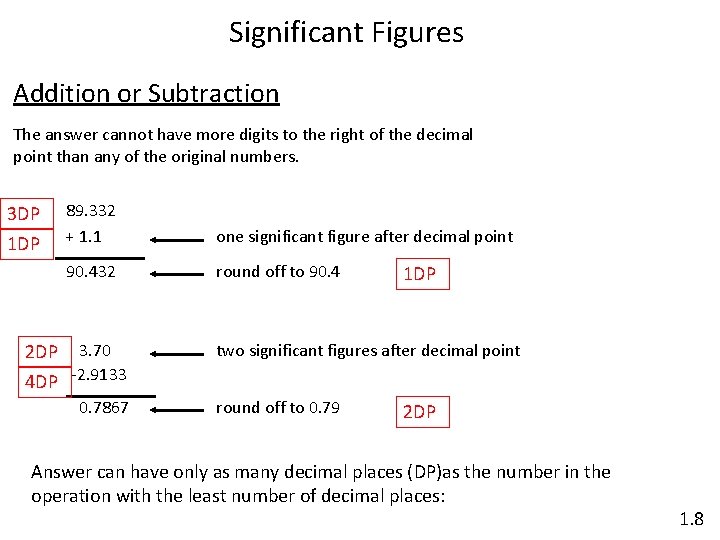
Significant Figures Addition or Subtraction The answer cannot have more digits to the right of the decimal point than any of the original numbers. 3 DP 1 DP 89. 332 + 1. 1 one significant figure after decimal point 90. 432 round off to 90. 4 2 DP 3. 70 4 DP -2. 9133 0. 7867 1 DP two significant figures after decimal point round off to 0. 79 2 DP Answer can have only as many decimal places (DP)as the number in the operation with the least number of decimal places: 1. 8

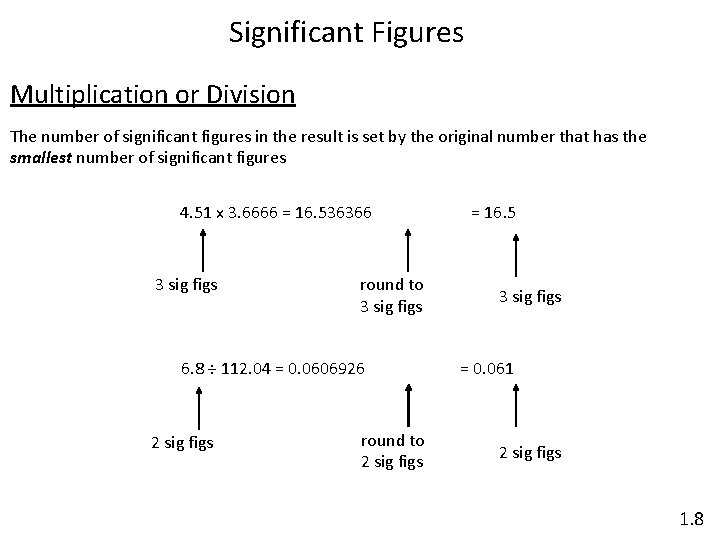
Significant Figures Multiplication or Division The number of significant figures in the result is set by the original number that has the smallest number of significant figures 4. 51 x 3. 6666 = 16. 536366 3 sig figs round to 3 sig figs 6. 8 ÷ 112. 04 = 0. 0606926 2 sig figs round to 2 sig figs = 16. 5 3 sig figs = 0. 061 2 sig figs 1. 8

Chapter 2

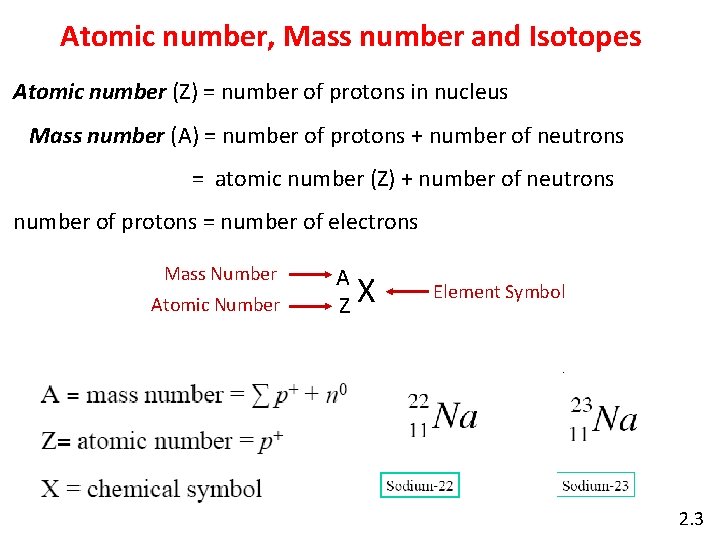
Atomic number, Mass number and Isotopes Atomic number (Z) = number of protons in nucleus Mass number (A) = number of protons + number of neutrons = atomic number (Z) + number of neutrons number of protons = number of electrons Mass Number Atomic Number A ZX Element Symbol 2. 3

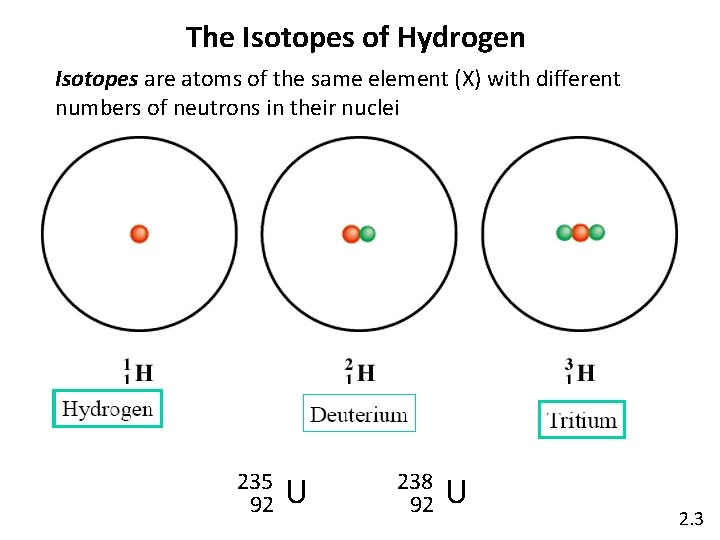
The Isotopes of Hydrogen Isotopes are atoms of the same element (X) with different numbers of neutrons in their nuclei 235 92 U 238 92 U 2. 3

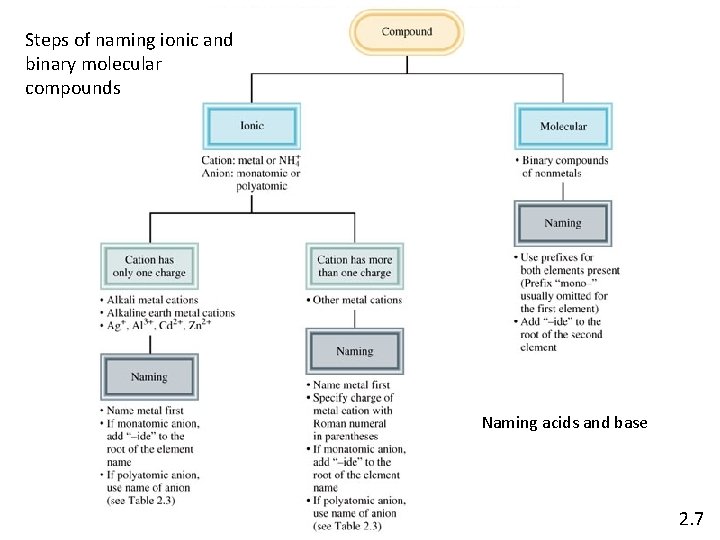
Steps of naming ionic and binary molecular compounds Naming acids and base 2. 7

Chapter 3

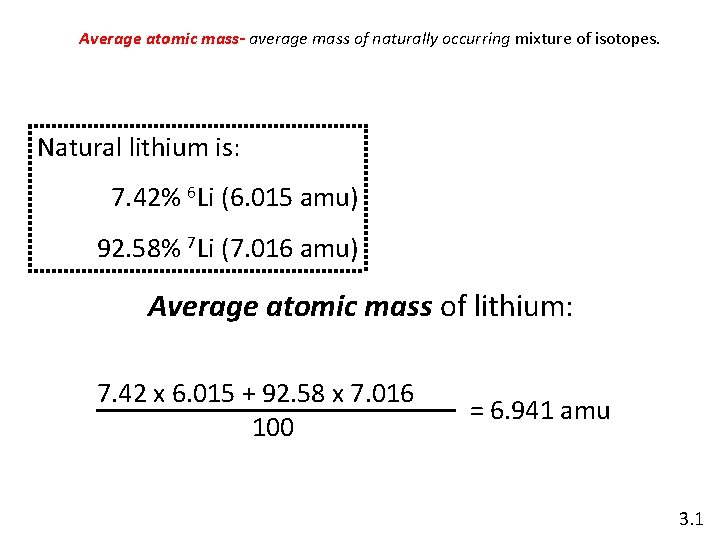
Average atomic mass- average mass of naturally occurring mixture of isotopes. Natural lithium is: 7. 42% 6 Li (6. 015 amu) 92. 58% 7 Li (7. 016 amu) Average atomic mass of lithium: 7. 42 x 6. 015 + 92. 58 x 7. 016 100 = 6. 941 amu 3. 1

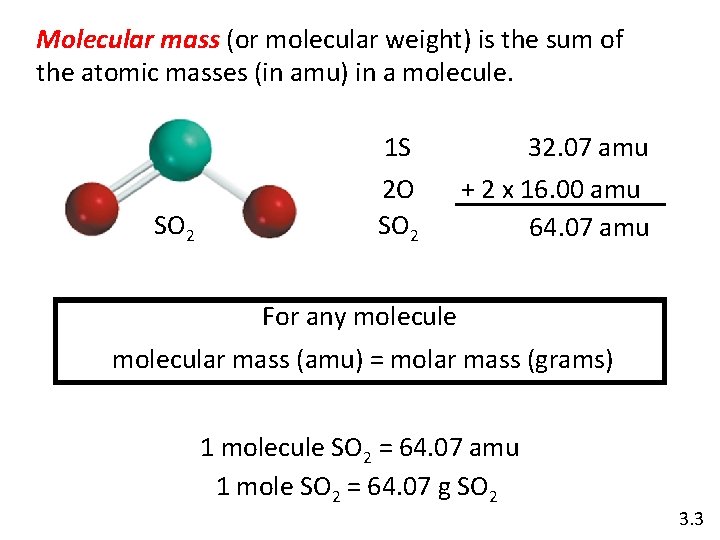
Molecular mass (or molecular weight) is the sum of the atomic masses (in amu) in a molecule. SO 2 1 S 32. 07 amu 2 O SO 2 + 2 x 16. 00 amu 64. 07 amu For any molecule molecular mass (amu) = molar mass (grams) 1 molecule SO 2 = 64. 07 amu 1 mole SO 2 = 64. 07 g SO 2 3. 3

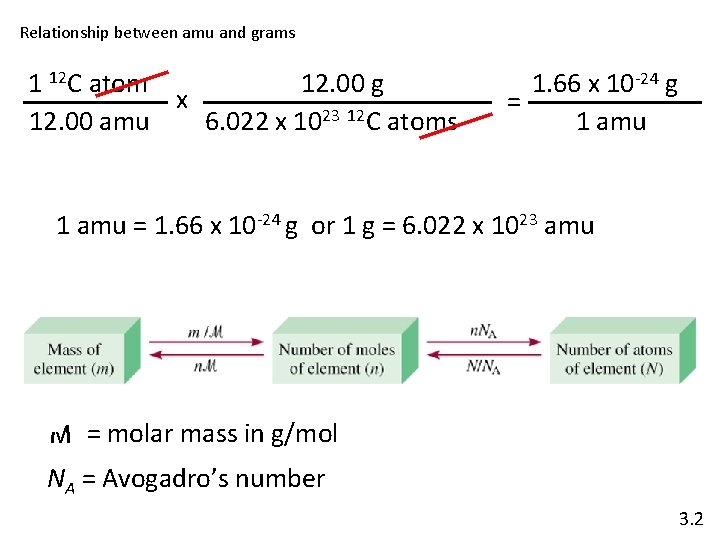
Relationship between amu and grams 1 12 C atom 12. 00 g x 12. 00 amu 6. 022 x 1023 12 C atoms 1. 66 x 10 -24 g = 1 amu = 1. 66 x 10 -24 g or 1 g = 6. 022 x 1023 amu M = molar mass in g/mol NA = Avogadro’s number 3. 2

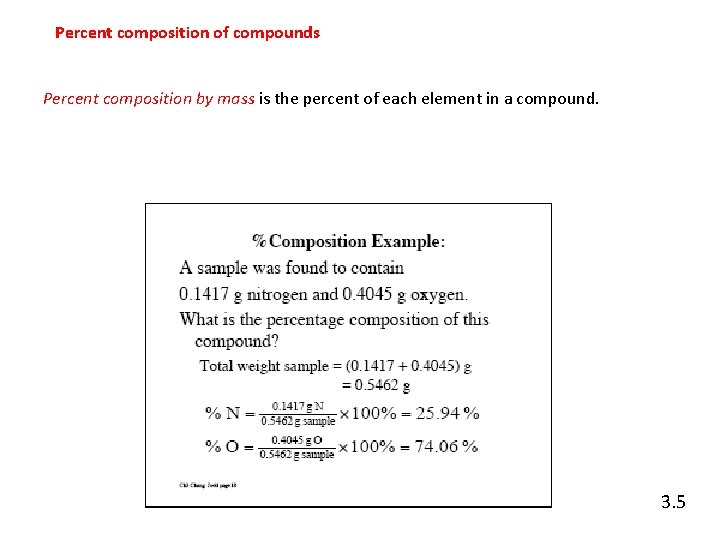
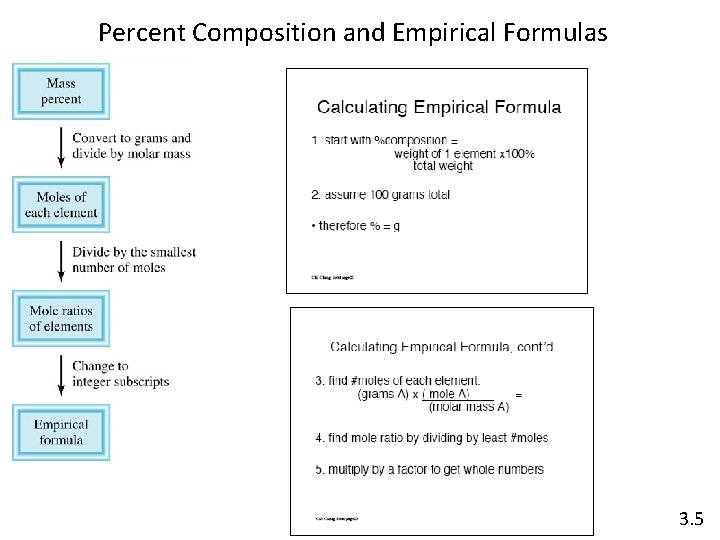
Percent composition of compounds Percent composition by mass is the percent of each element in a compound. 3. 5

Percent Composition and Empirical Formulas 3. 5

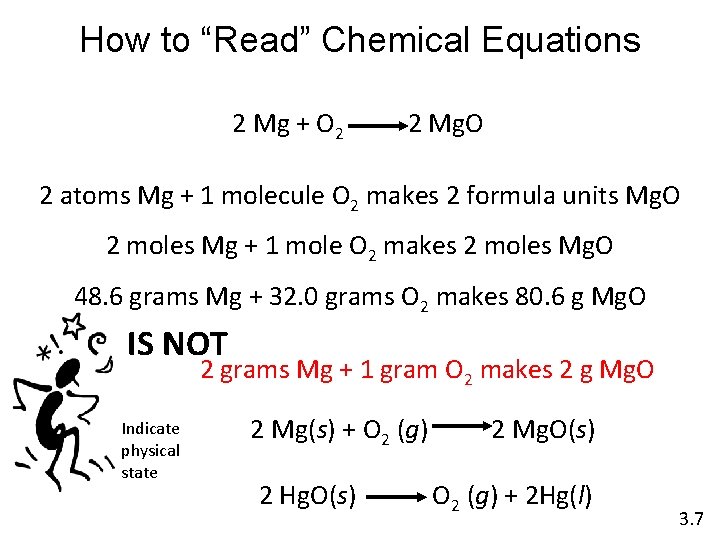
How to “Read” Chemical Equations 2 Mg + O 2 2 Mg. O 2 atoms Mg + 1 molecule O 2 makes 2 formula units Mg. O 2 moles Mg + 1 mole O 2 makes 2 moles Mg. O 48. 6 grams Mg + 32. 0 grams O 2 makes 80. 6 g Mg. O IS NOT 2 grams Mg + 1 gram O 2 makes 2 g Mg. O Indicate physical state 2 Mg(s) + O 2 (g) 2 Hg. O(s) 2 Mg. O(s) O 2 (g) + 2 Hg(l) 3. 7

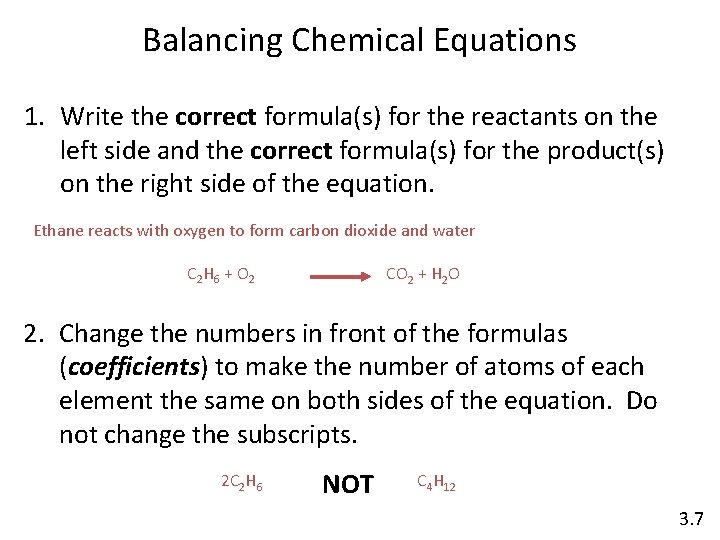
Balancing Chemical Equations 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide and water C 2 H 6 + O 2 CO 2 + H 2 O 2. Change the numbers in front of the formulas (coefficients) to make the number of atoms of each element the same on both sides of the equation. Do not change the subscripts. 2 C 2 H 6 NOT C 4 H 12 3. 7

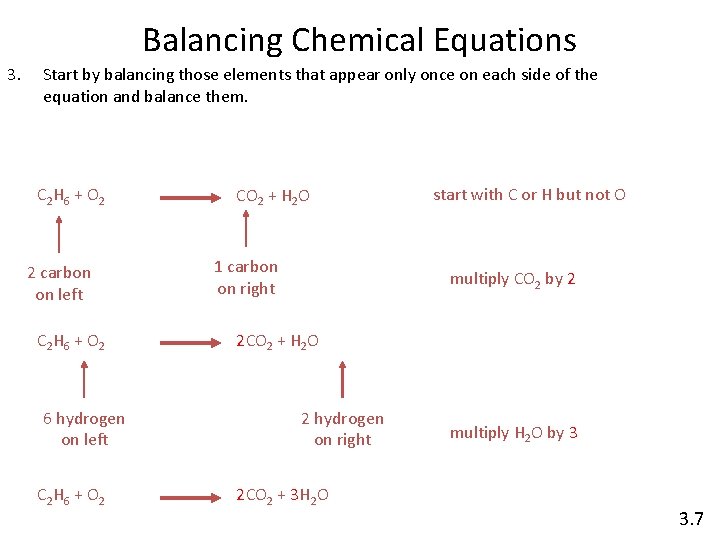
Balancing Chemical Equations 3. Start by balancing those elements that appear only once on each side of the equation and balance them. C 2 H 6 + O 2 2 carbon on left C 2 H 6 + O 2 6 hydrogen on left C 2 H 6 + O 2 CO 2 + H 2 O 1 carbon on right start with C or H but not O multiply CO 2 by 2 2 CO 2 + H 2 O 2 hydrogen on right 2 CO 2 + 3 H 2 O multiply H 2 O by 3 3. 7

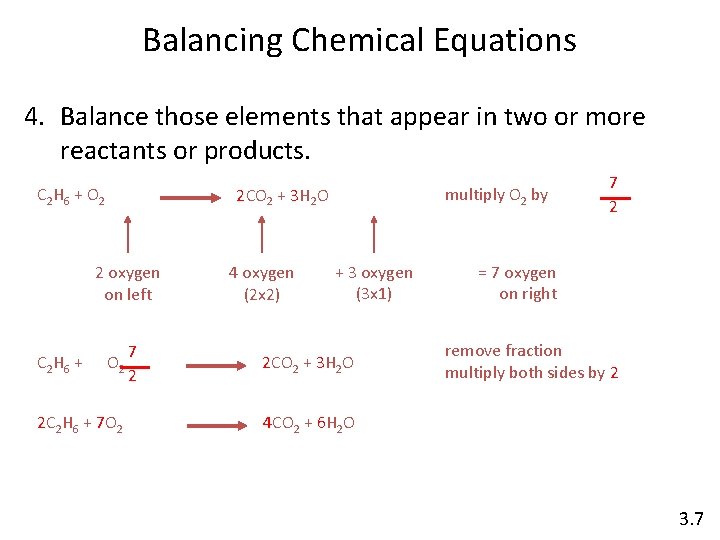
Balancing Chemical Equations 4. Balance those elements that appear in two or more reactants or products. C 2 H 6 + O 2 2 oxygen on left C 2 H 6 + multiply O 2 by 2 CO 2 + 3 H 2 O O 2 2 C 2 H 6 + 7 O 2 7 2 4 oxygen (2 x 2) + 3 oxygen (3 x 1) 2 CO 2 + 3 H 2 O 7 2 = 7 oxygen on right remove fraction multiply both sides by 2 4 CO 2 + 6 H 2 O 3. 7

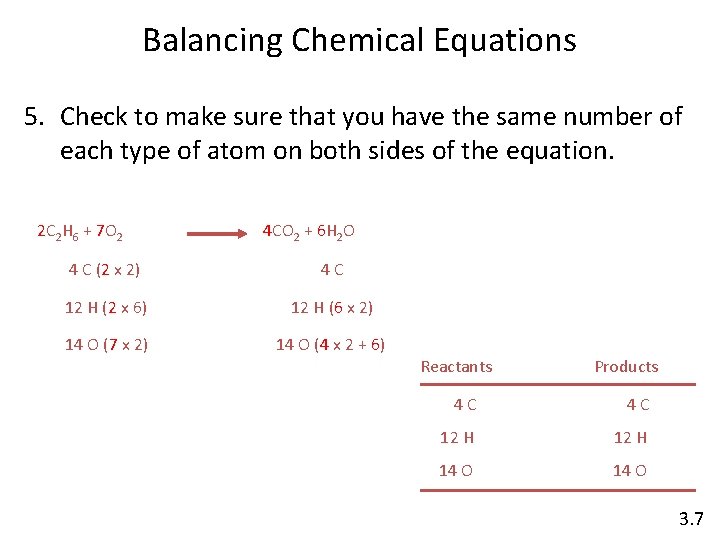
Balancing Chemical Equations 5. Check to make sure that you have the same number of each type of atom on both sides of the equation. 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O 4 C (2 x 2) 4 C 12 H (2 x 6) 12 H (6 x 2) 14 O (7 x 2) 14 O (4 x 2 + 6) Reactants Products 4 C 4 C 12 H 14 O 3. 7

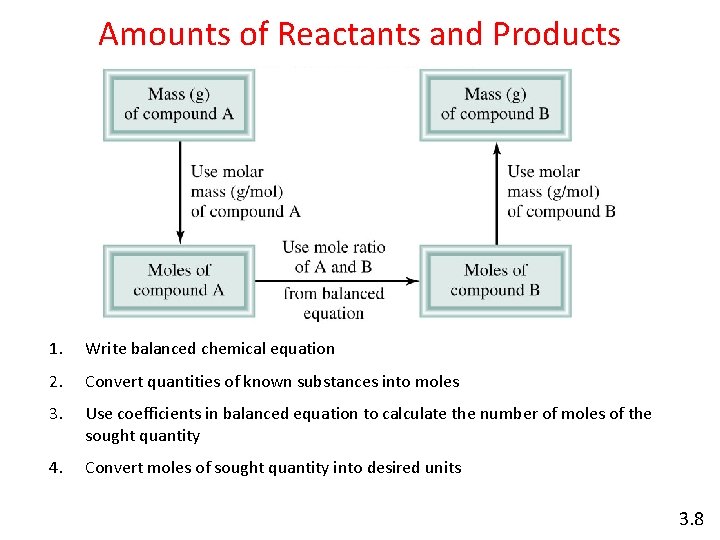
Amounts of Reactants and Products 1. Write balanced chemical equation 2. Convert quantities of known substances into moles 3. Use coefficients in balanced equation to calculate the number of moles of the sought quantity 4. Convert moles of sought quantity into desired units 3. 8

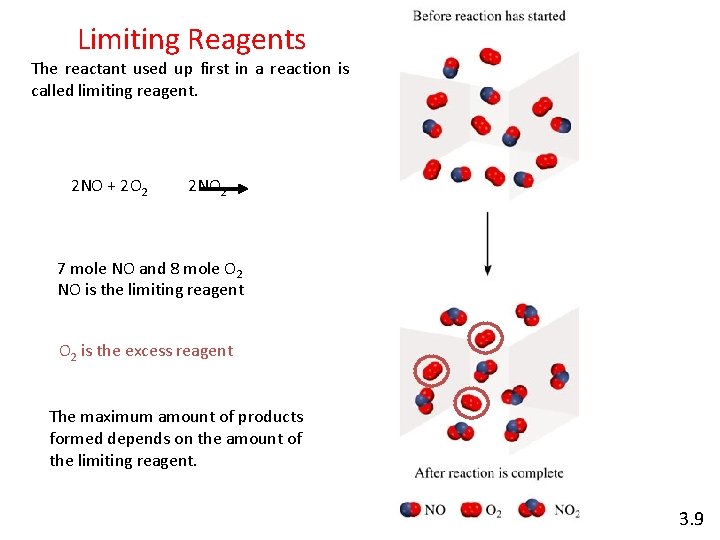
Limiting Reagents The reactant used up first in a reaction is called limiting reagent. 2 NO + 2 O 2 2 NO 2 7 mole NO and 8 mole O 2 NO is the limiting reagent O 2 is the excess reagent The maximum amount of products formed depends on the amount of the limiting reagent. 3. 9

Chapter 4

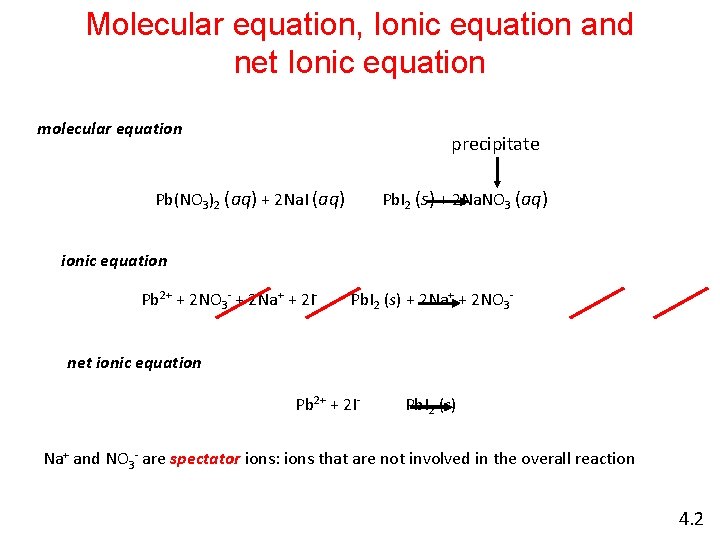
Molecular equation, Ionic equation and net Ionic equation molecular equation precipitate Pb(NO 3)2 (aq) + 2 Na. I (aq) Pb. I 2 (s) + 2 Na. NO 3 (aq) ionic equation Pb 2+ + 2 NO 3 - + 2 Na+ + 2 I- Pb. I 2 (s) + 2 Na+ + 2 NO 3 - net ionic equation Pb 2+ + 2 I- Pb. I 2 (s) Na+ and NO 3 - are spectator ions: ions that are not involved in the overall reaction 4. 2

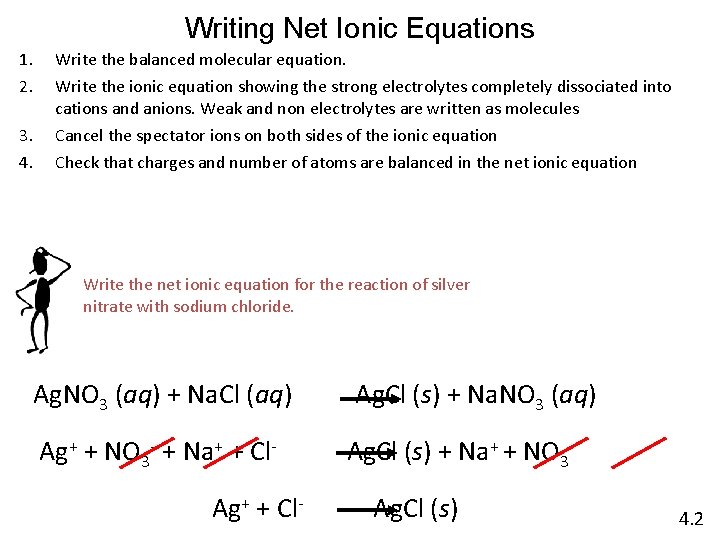
Writing Net Ionic Equations 1. 2. 3. 4. Write the balanced molecular equation. Write the ionic equation showing the strong electrolytes completely dissociated into cations and anions. Weak and non electrolytes are written as molecules Cancel the spectator ions on both sides of the ionic equation Check that charges and number of atoms are balanced in the net ionic equation Write the net ionic equation for the reaction of silver nitrate with sodium chloride. Ag. NO 3 (aq) + Na. Cl (aq) Ag+ + NO 3 - + Na+ + Cl. Ag+ + Cl- Ag. Cl (s) + Na. NO 3 (aq) Ag. Cl (s) + Na+ + NO 3 Ag. Cl (s) 4. 2

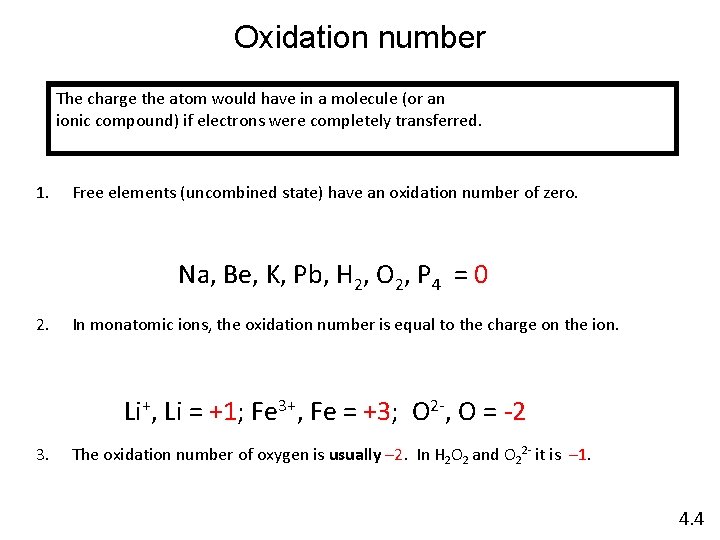
Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na, Be, K, Pb, H 2, O 2, P 4 = 0 2. In monatomic ions, the oxidation number is equal to the charge on the ion. Li+, Li = +1; Fe 3+, Fe = +3; O 2 -, O = -2 3. The oxidation number of oxygen is usually – 2. In H 2 O 2 and O 22 - it is – 1. 4. 4

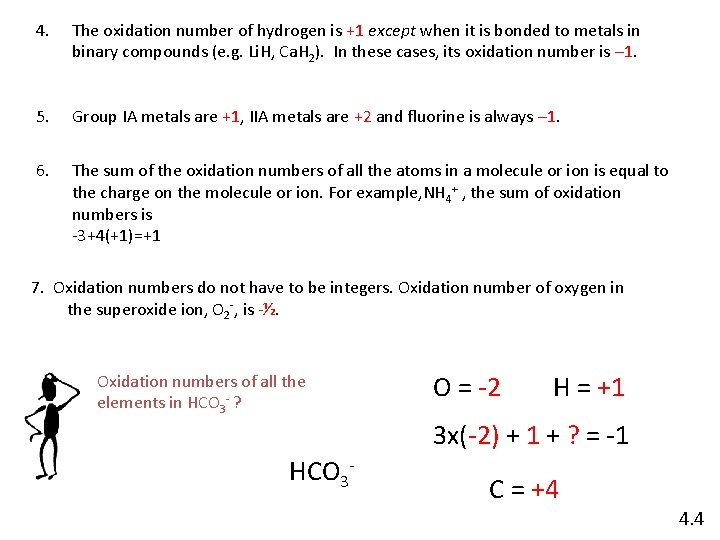
4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds (e. g. Li. H, Ca. H 2). In these cases, its oxidation number is – 1. 5. Group IA metals are +1, IIA metals are +2 and fluorine is always – 1. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. For example, NH 4+ , the sum of oxidation numbers is -3+4(+1)=+1 7. Oxidation numbers do not have to be integers. Oxidation number of oxygen in the superoxide ion, O 2 -, is -½. Oxidation numbers of all the elements in HCO 3 - ? O = -2 H = +1 3 x(-2) + 1 + ? = -1 HCO 3 - C = +4 4. 4

5. Disproportionation reaction

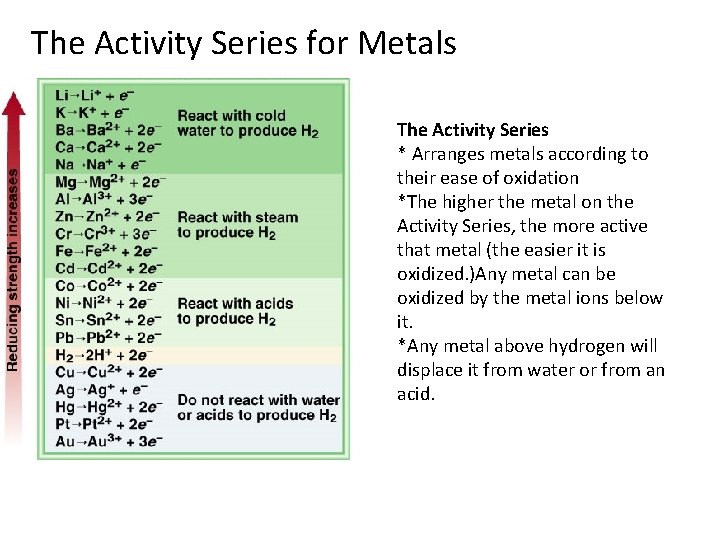
The Activity Series for Metals The Activity Series * Arranges metals according to their ease of oxidation *The higher the metal on the Activity Series, the more active that metal (the easier it is oxidized. )Any metal can be oxidized by the metal ions below it. *Any metal above hydrogen will displace it from water or from an acid.

3. Halogen Displacement F 2 > Cl 2 > Br 2 > I 2 F 2 is the greatest oxidizing halogen I 2 is the least oxidizing halogen Example: -1 0 2 Br- + Cl 2 --> 2 Cl- + Br 2 + Cl- --> no reaction (NR)

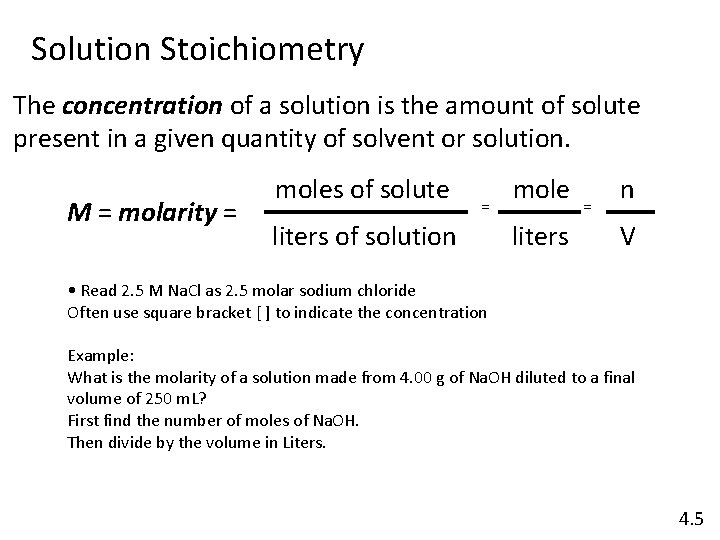
Solution Stoichiometry The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. M = molarity = moles of solute = liters of solution mole liters = n V • Read 2. 5 M Na. Cl as 2. 5 molar sodium chloride Often use square bracket [ ] to indicate the concentration Example: What is the molarity of a solution made from 4. 00 g of Na. OH diluted to a final volume of 250 m. L? First find the number of moles of Na. OH. Then divide by the volume in Liters. 4. 5


Chapter 5

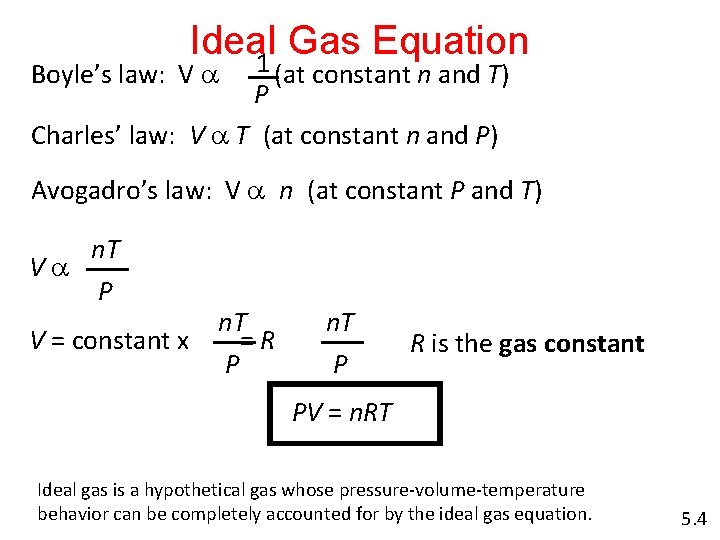
Ideal Gas Equation 1 Boyle’s law: V a (at constant n and T) P Charles’ law: V a T (at constant n and P) Avogadro’s law: V a n (at constant P and T) n. T Va P V = constant x n. T =R P n. T P R is the gas constant PV = n. RT Ideal gas is a hypothetical gas whose pressure-volume-temperature behavior can be completely accounted for by the ideal gas equation. 5. 4

The conditions 0 0 C and 1 atm are called standard temperature and pressure (STP). Experiments show that at STP, 1 mole of an ideal gas occupies 22. 414 L. Molar volume of gas • 1 mole of gas at STP = 22. 4 Liters • Example: 2 moles of gas at STP = 44. 8 L PV = n. RT (1 atm)(22. 414 L) PV R= = n. T (1 mol)(273. 15 K) R = 0. 082057 L • atm / (mol • K)=8. 314 J/(K·mol) = 8. 314 L·k. Pa/(K·mol) In calculation, the units of R must match those for P, V, T and n. 5. 4

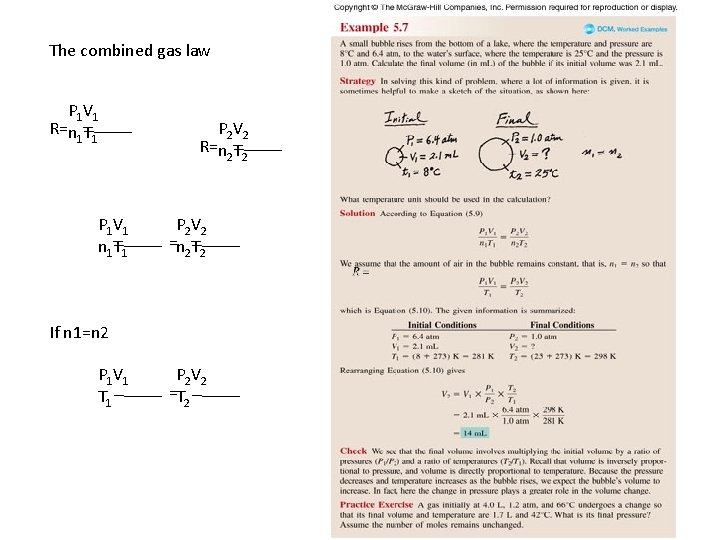
The combined gas law P 1 V 1 R=n 1 T 1 P 1 V 1 n 1 T 1 P 2 V 2 R=n 2 T 2 P 2 V 2 =n 2 T 2 If n 1=n 2 P 1 V 1 T 1 P 2 V 2 =T 2

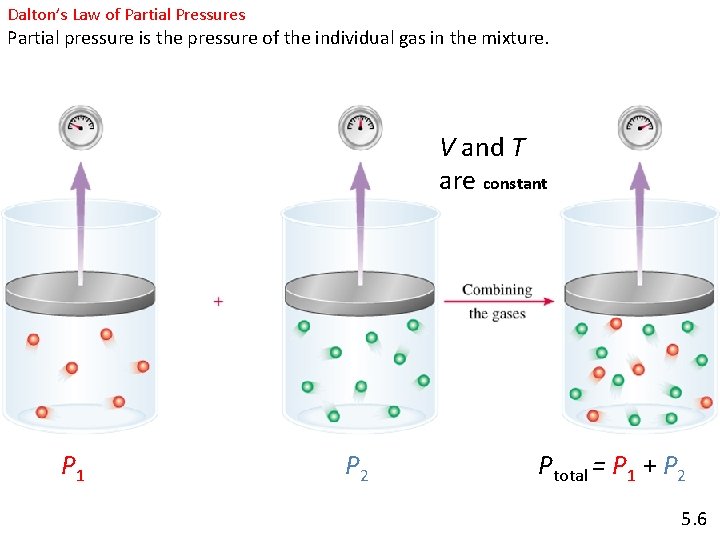
Dalton’s Law of Partial Pressures Partial pressure is the pressure of the individual gas in the mixture. V and T are constant P 1 P 2 Ptotal = P 1 + P 2 5. 6

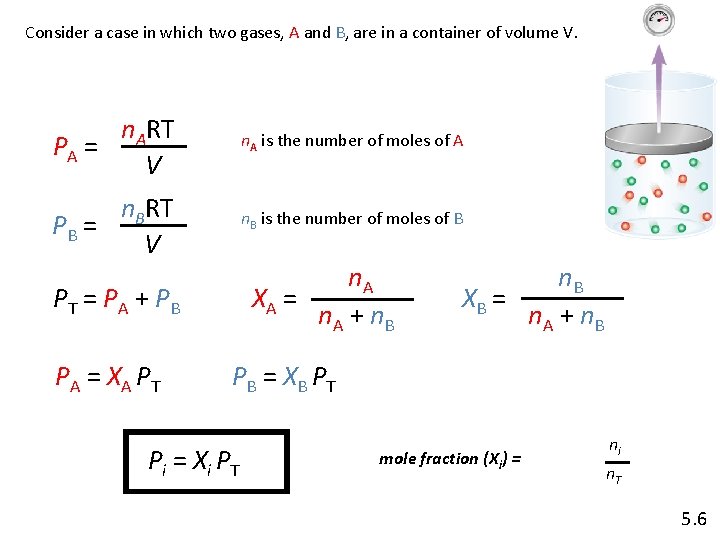
Consider a case in which two gases, A and B, are in a container of volume V. n. ART PA = V n. A is the number of moles of A n. BRT PB = V n. B is the number of moles of B n. A XA = n. A + n. B PT = PA + PB P A = XA P T n. B XB = n. A + n. B P B = XB P T P i = Xi P T mole fraction (Xi) = ni n. T 5. 6

Chapter 6

Another form of the first law for DEsystem DE = q + w DE is the change in internal energy of a system q is the heat exchange between the system and the surroundings w is the work done on (or by) the system w = -PDV when a gas expands against a constant external pressure 6. 3

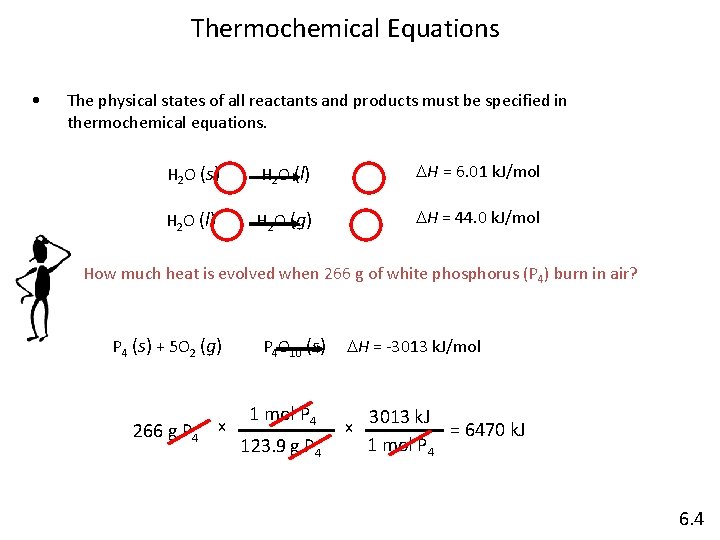
Thermochemical Equations • The physical states of all reactants and products must be specified in thermochemical equations. H 2 O (s) H 2 O (l) DH = 6. 01 k. J/mol H 2 O (l) H 2 O (g) DH = 44. 0 k. J/mol How much heat is evolved when 266 g of white phosphorus (P 4) burn in air? P 4 (s) + 5 O 2 (g) 266 g P 4 x P 4 O 10 (s) 1 mol P 4 123. 9 g P 4 DH = -3013 k. J/mol x 3013 k. J 1 mol P 4 = 6470 k. J 6. 4

Consider the reaction below: 2 CH 3 OH(l) + 3 O 2(g) 4 H 2 O(l) + 2 CO 2(g) ∆H = -1452. 8 k. J/mol What is the value of ∆H for the reaction of 8 H 2 O(l) + 4 CO 2(g) 4 CH 3 OH (l) + 6 O 2 (g)? If you reverse a reaction, the sign of ΔH changes. If you multiply both sides of the equation by a factor n, then ∆H must change by the same factor n. ∆H = 2*(+1452. 8 k. J/mol) = +2905. 6 KJ/mol Practice problem of chap 6: Q 2

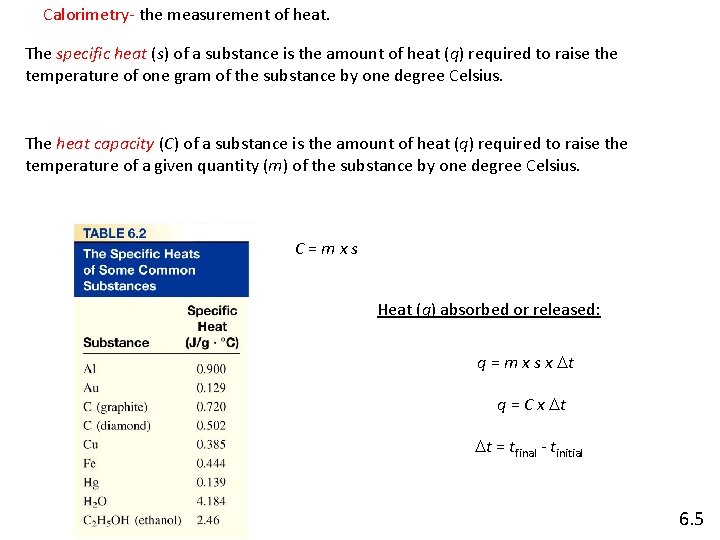
Calorimetry- the measurement of heat. The specific heat (s) of a substance is the amount of heat (q) required to raise the temperature of one gram of the substance by one degree Celsius. The heat capacity (C) of a substance is the amount of heat (q) required to raise the temperature of a given quantity (m) of the substance by one degree Celsius. C=mxs Heat (q) absorbed or released: q = m x s x Dt q = C x Dt Dt = tfinal - tinitial 6. 5

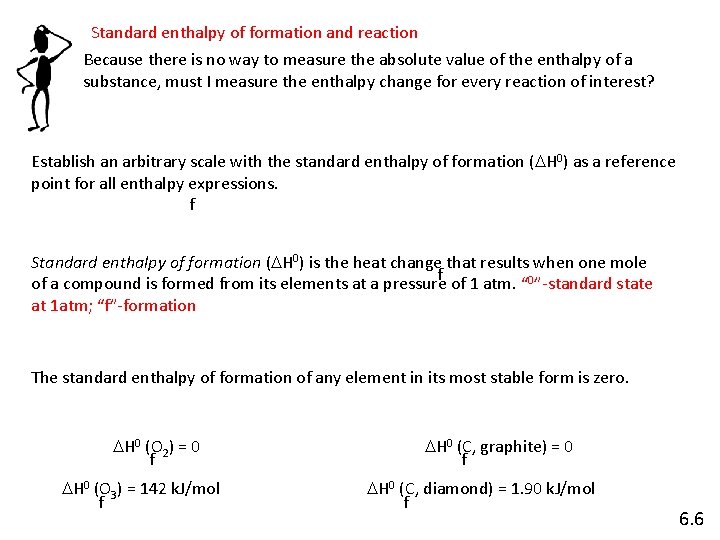
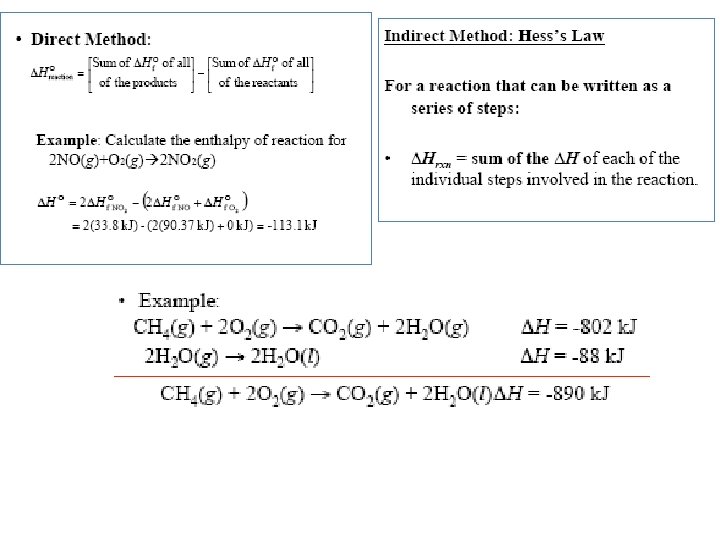
Standard enthalpy of formation and reaction Because there is no way to measure the absolute value of the enthalpy of a substance, must I measure the enthalpy change for every reaction of interest? Establish an arbitrary scale with the standard enthalpy of formation (DH 0) as a reference point for all enthalpy expressions. f Standard enthalpy of formation (DH 0) is the heat change that results when one mole f of a compound is formed from its elements at a pressure of 1 atm. “ 0”-standard state at 1 atm; “f”-formation The standard enthalpy of formation of any element in its most stable form is zero. DH 0 (O 2) = 0 f DH 0 (O 3) = 142 k. J/mol f DH 0 (C, graphite) = 0 f DH 0 (C, diamond) = 1. 90 k. J/mol f 6. 6

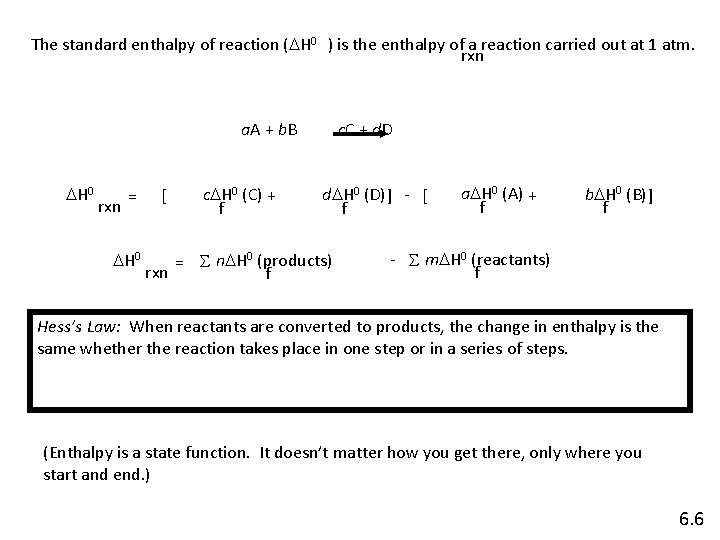
The standard enthalpy of reaction (DH 0 ) is the enthalpy of a reaction carried out at 1 atm. rxn a. A + b. B DH 0 rxn = DH 0 [ c. DH 0 (C) + f c. C + d. DH 0 (D) ] - [ f = S n. DH 0 (products) rxn f a. DH 0 (A) + f b. DH 0 (B) ] f - S m. DH 0 (reactants) f Hess’s Law: When reactants are converted to products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps. (Enthalpy is a state function. It doesn’t matter how you get there, only where you start and end. ) 6. 6


Chapter 7

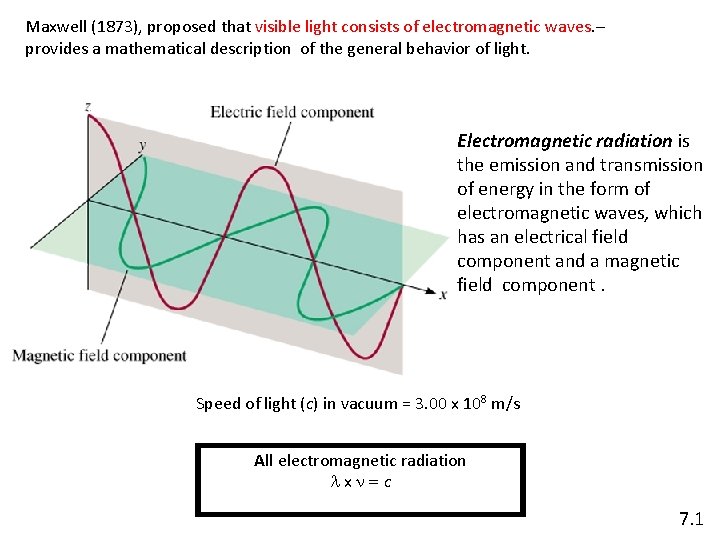
Maxwell (1873), proposed that visible light consists of electromagnetic waves. – provides a mathematical description of the general behavior of light. Electromagnetic radiation is the emission and transmission of energy in the form of electromagnetic waves, which has an electrical field component and a magnetic field component. Speed of light (c) in vacuum = 3. 00 x 108 m/s All electromagnetic radiation lxn=c 7. 1

Energy of light: Quantum = packet of energy Photon = packet of light • E=hn Planck’s constant (h) h = 6. 63 x 10 -34 J • s

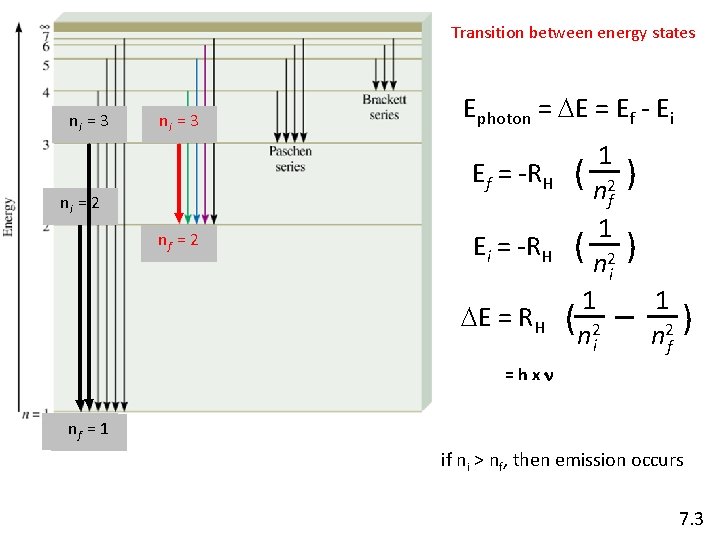
Transition between energy states ni = 3 ni = 2 nf = 2 Ephoton = DE = Ef - Ei 1 Ef = -RH ( 2 nf 1 Ei = -RH ( 2 ni 1 DE = RH ( 2 ni ) ) 1 ) n 2 f =hxn nnf =f =11 if ni > nf, then emission occurs 7. 3

Quantum Mechanical Description of the distribution of electrons in the atom: • 4 quantum numbers: n (principal quantum number) l (angular momentum quantum number) ml (magnetic quantum number) ms (spin quantum number) Allowed values: • n = 1, 2, 3 …, n • l = 0, 1, 2, …, (n-1) • ml = (-l, …, 0. …+l) • ms = +1/2, -1/2

Electron Configurations Periods 1, 2, and 3 Three rules: 1. Electrons fill orbitals starting with lowest n and moving upwards (Aufbau principle: Fill up electrons in lowest energy orbitals ) 2. No more than two electrons can be placed in each orbital. No two electrons can fill one orbital with the same spin (Pauli exclusion principle: no two electrons in an atom can have the same four quantum numbers. ) 3. For degenerate orbitals, electrons fill each orbital singly before any orbital gets a second electron (Hund’s rule: The most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins ) Period 4 and Beyond the d orbitals begin to fill

Chapter 8
![Electron Configurations of Cations and Anions Of Representative Elements Na Ne3 s 1 Na Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Na+](https://slidetodoc.com/presentation_image_h2/6632e5656b2d9eaebbc77ba14d846ad9/image-55.jpg)
Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Na+ [Ne] Ca [Ar]4 s 2 Ca 2+ [Ar] Al [Ne]3 s 23 p 1 Al 3+ [Ne] Atoms gain electrons so that anion has a noble-gas outer electron configuration. Atoms lose electrons so that cation has a noble-gas outer electron configuration. Remember: electrons are first removed from orbitals with the highest principal quantum number. H 1 s 1 H- 1 s 2 or [He] F 1 s 22 p 5 F- 1 s 22 p 6 or [Ne] O 1 s 22 p 4 O 2 - 1 s 22 p 6 or [Ne] N 1 s 22 p 3 N 3 - 1 s 22 p 6 or [Ne] 8. 2

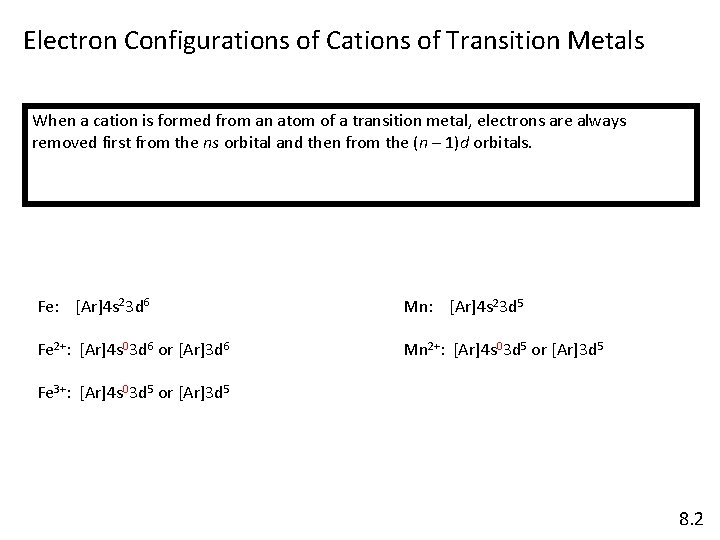
Electron Configurations of Cations of Transition Metals When a cation is formed from an atom of a transition metal, electrons are always removed first from the ns orbital and then from the (n – 1)d orbitals. Fe: [Ar]4 s 23 d 6 Mn: [Ar]4 s 23 d 5 Fe 2+: [Ar]4 s 03 d 6 or [Ar]3 d 6 Mn 2+: [Ar]4 s 03 d 5 or [Ar]3 d 5 Fe 3+: [Ar]4 s 03 d 5 or [Ar]3 d 5 8. 2

Periodic Variation in Physical Properties

8. 3

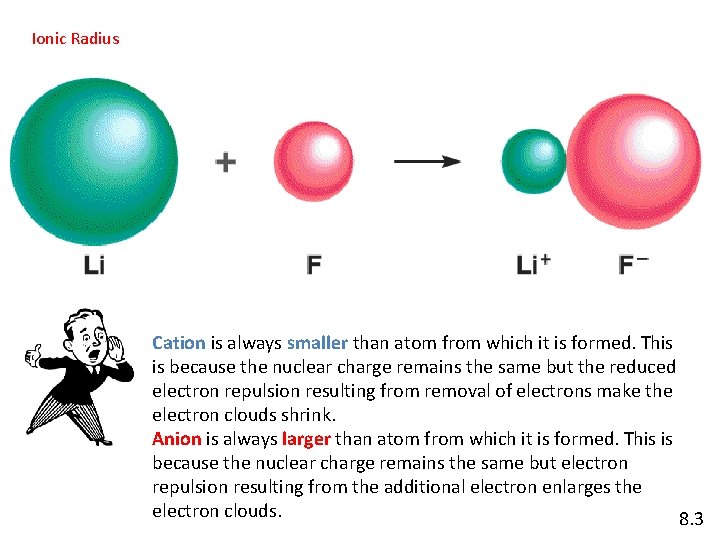
Ionic Radius Cation is always smaller than atom from which it is formed. This is because the nuclear charge remains the same but the reduced electron repulsion resulting from removal of electrons make the electron clouds shrink. Anion is always larger than atom from which it is formed. This is because the nuclear charge remains the same but electron repulsion resulting from the additional electron enlarges the electron clouds. 8. 3

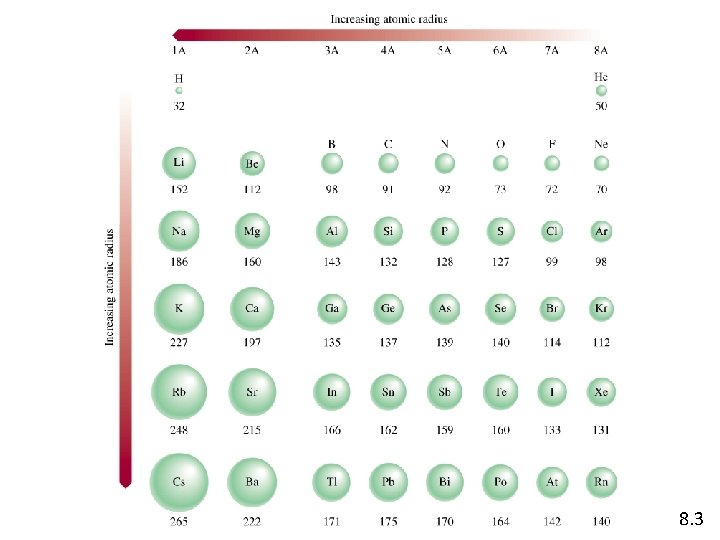
• From top to bottom, both the atomic and ionic radius increase within group. • Across a period the anions are usually larger than cations. • For ions derived from different groups, size comparison is meaningful only if the ions are isoelectronic. E. g. Na+ (Z=11) is smaller than F- (Z=9). The larger effective charge results in a smaller radius. Radius of tripositive ions < dipositive ions<unipositive ions Al 3+ <Mg 2+ <Na+ Radius of uninegative ions < dinegative ions O 2 ->F-

General Trend in First Ionization Energies Increasing First Ionization Energy 8. 4

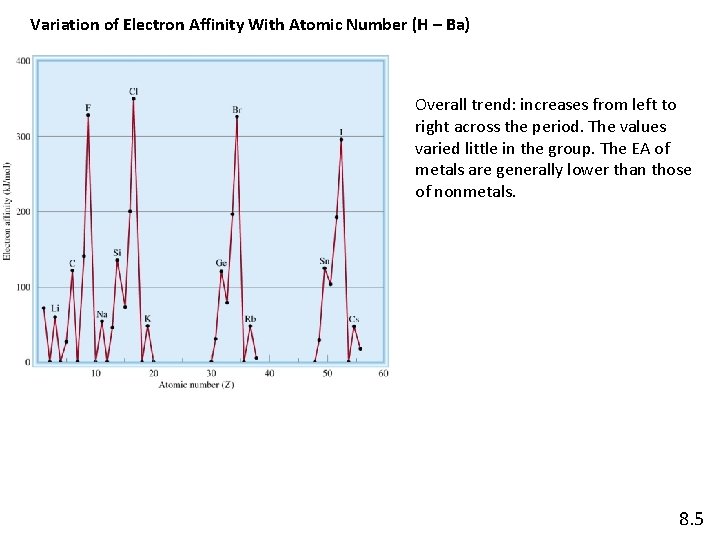
Variation of Electron Affinity With Atomic Number (H – Ba) Overall trend: increases from left to right across the period. The values varied little in the group. The EA of metals are generally lower than those of nonmetals. 8. 5

Chapter 9

The Octet Rule Atoms tend to gain, lose, or share electrons until they are surrounded by 8 valence electrons. An octet = 8 electrons = 4 pairs of electrons = noble gas arrangement = very stable Exception: H = 2 electrons only Example: Na [Ne]3 s 1 loses 1 e----> Na+ [Ne] Cl [Ne]3 s 23 p 5 gains 1 e- --> Cl- [Ar]

Writing Lewis Structures 1. Draw skeletal structure of compound showing what atoms are bonded to each other. Put least electronegative element in the center. Arrange atoms & connect with single bonds – Single bond = 2 electrons 2. Count total number of valence e-. Add 1 for each negative charge. Subtract 1 for each positive charge. 3. Complete an octet for atoms bonded to the central atom except hydrogen (H=2). Then add leftover electrons to central atom. Electrons belonging to the central or surrounding atoms must be shown as lone pairs if they are not involved in bonding. 4. If the central atom has fewer than 8 electrons, try adding double and triple bonds on central atom as needed. 9. 6

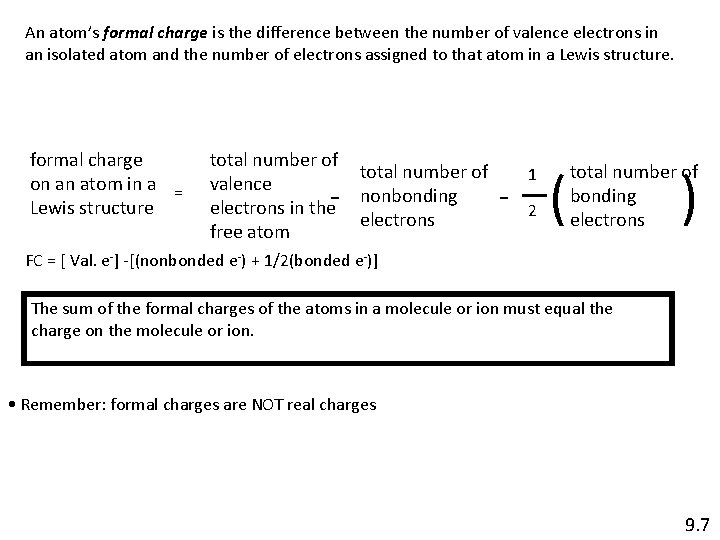
An atom’s formal charge is the difference between the number of valence electrons in an isolated atom and the number of electrons assigned to that atom in a Lewis structure. formal charge on an atom in a = Lewis structure total number of valence electrons in the free atom - total number of nonbonding electrons - 1 2 ( ) total number of bonding electrons FC = [ Val. e-] -[(nonbonded e-) + 1/2(bonded e-)] The sum of the formal charges of the atoms in a molecule or ion must equal the charge on the molecule or ion. • Remember: formal charges are NOT real charges 9. 7

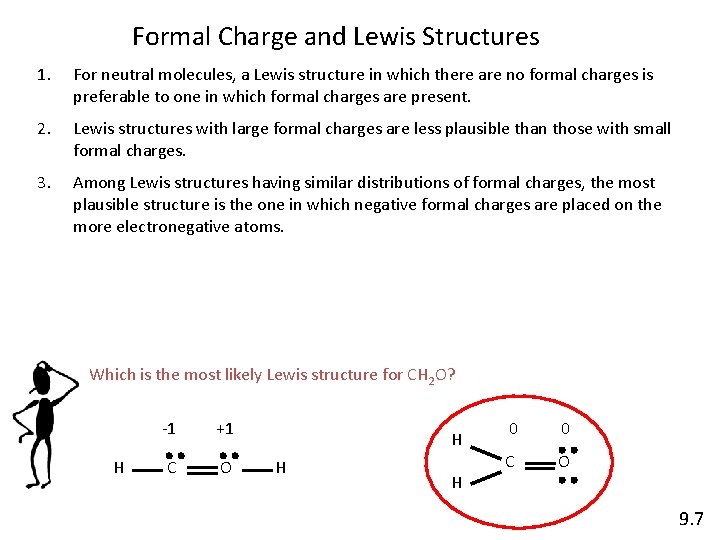
Formal Charge and Lewis Structures 1. For neutral molecules, a Lewis structure in which there are no formal charges is preferable to one in which formal charges are present. 2. Lewis structures with large formal charges are less plausible than those with small formal charges. 3. Among Lewis structures having similar distributions of formal charges, the most plausible structure is the one in which negative formal charges are placed on the more electronegative atoms. Which is the most likely Lewis structure for CH 2 O? H -1 +1 C O H H H 0 0 C O 9. 7

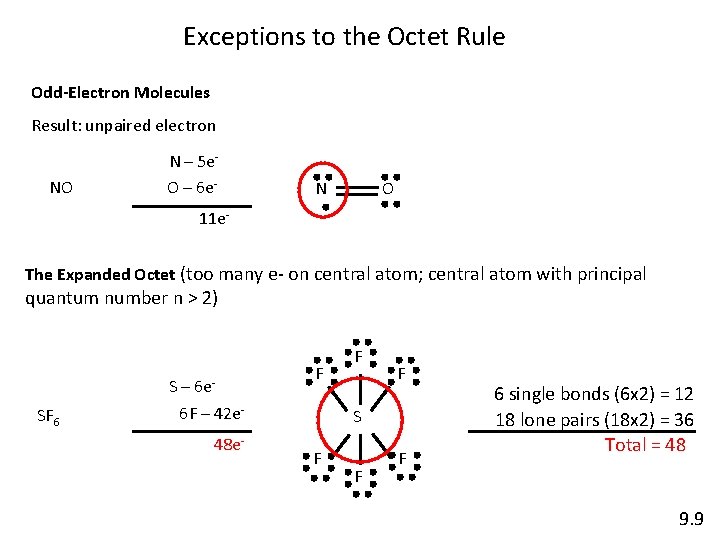
Exceptions to the Octet Rule Odd-Electron Molecules Result: unpaired electron NO N – 5 e. O – 6 e- N O 11 e. The Expanded Octet (too many e- on central atom; central atom with principal quantum number n > 2) 6 e- SF 6 S– 6 F – 42 e 48 e- F F F S F F F 6 single bonds (6 x 2) = 12 18 lone pairs (18 x 2) = 36 Total = 48 9. 9

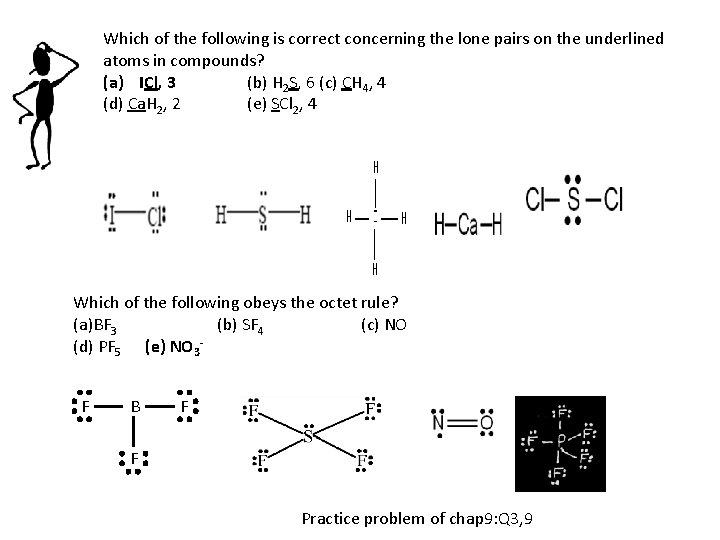
Which of the following is correct concerning the lone pairs on the underlined atoms in compounds? (a) ICl, 3 (b) H 2 S, 6 (c) CH 4, 4 (d) Ca. H 2, 2 (e) SCl 2, 4 Which of the following obeys the octet rule? (a)BF 3 (b) SF 4 (c) NO (d) PF 5 (e) NO 3 F B F F Practice problem of chap 9: Q 3, 9

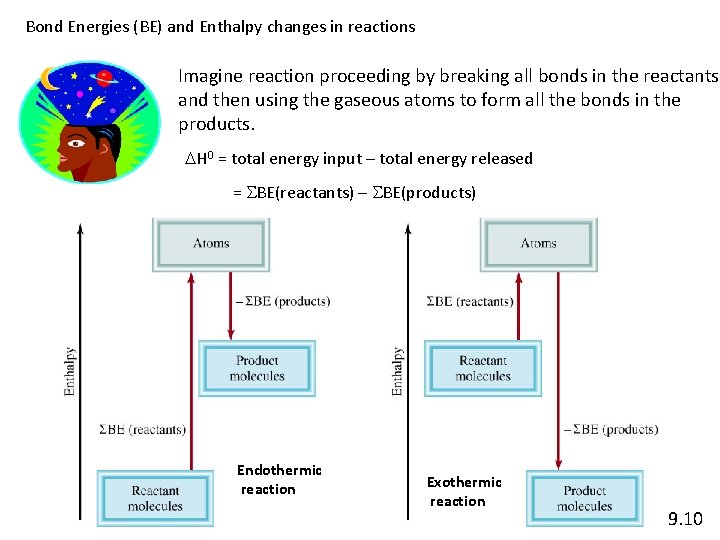
Bond Energies (BE) and Enthalpy changes in reactions Imagine reaction proceeding by breaking all bonds in the reactants and then using the gaseous atoms to form all the bonds in the products. DH 0 = total energy input – total energy released = SBE(reactants) – SBE(products) Endothermic reaction Exothermic reaction 9. 10

Chapter 10

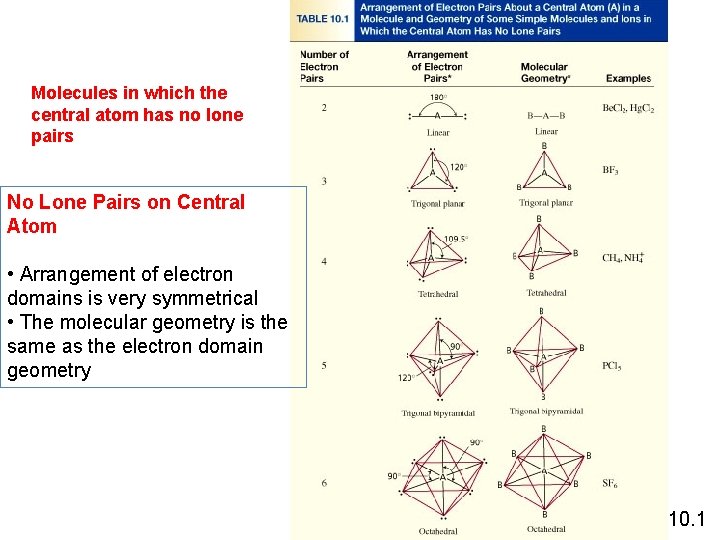
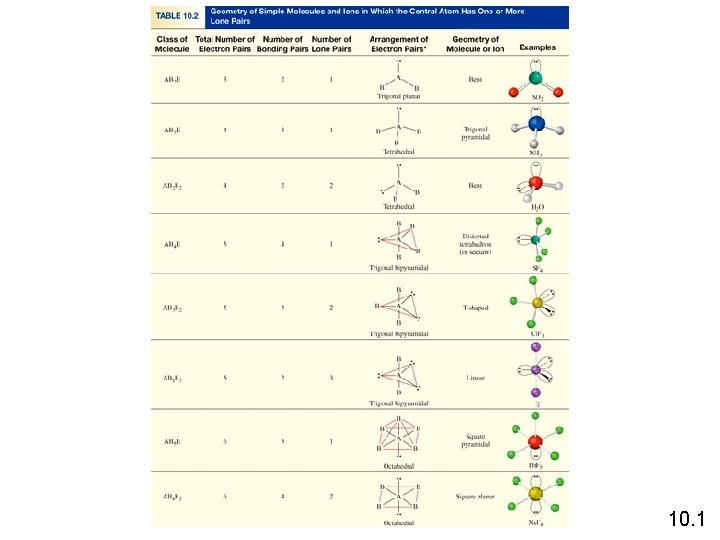
Molecules in which the central atom has no lone pairs No Lone Pairs on Central Atom • Arrangement of electron domains is very symmetrical • The molecular geometry is the same as the electron domain geometry 10. 1

Steps to Assign Molecular Geometry • Draw Lewis structure • Count # of electron domains around the Central atom(treat double and triple bonds as though there were single bond) • Assign electron domain geometry • Finally assign the molecular shape – based on the arrangement of the atoms – not the arrangement of the domains No Lone Pairs on Central Atom • Arrangement of electron domains is very symmetrical • The molecular geometry is the same as the electron domain geometry

10. 1

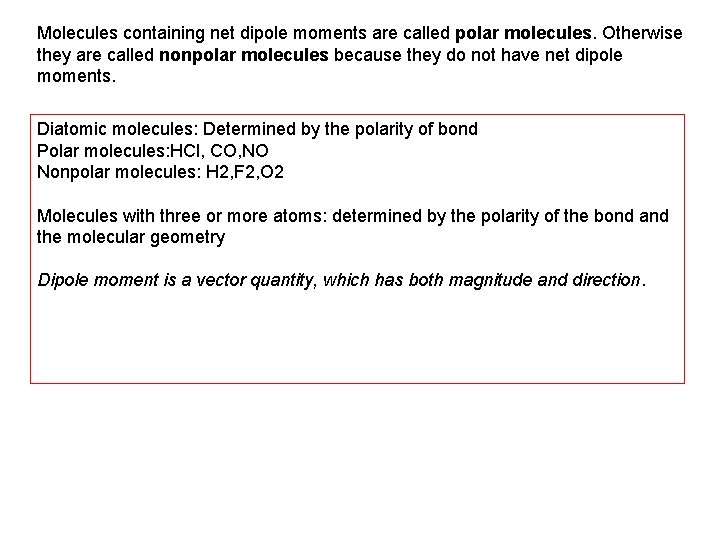
Molecules containing net dipole moments are called polar molecules. Otherwise they are called nonpolar molecules because they do not have net dipole moments. Diatomic molecules: Determined by the polarity of bond Polar molecules: HCl, CO, NO Nonpolar molecules: H 2, F 2, O 2 Molecules with three or more atoms: determined by the polarity of the bond and the molecular geometry Dipole moment is a vector quantity, which has both magnitude and direction.

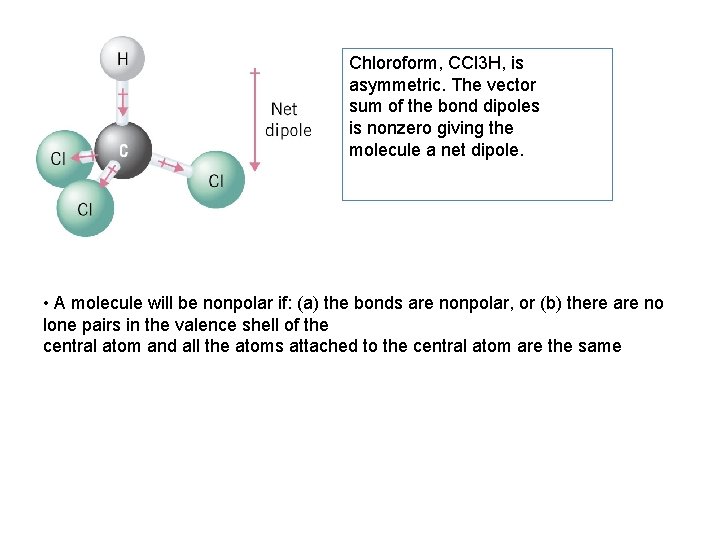
Chloroform, CCl 3 H, is asymmetric. The vector sum of the bond dipoles is nonzero giving the molecule a net dipole. • A molecule will be nonpolar if: (a) the bonds are nonpolar, or (b) there are no lone pairs in the valence shell of the central atom and all the atoms attached to the central atom are the same

10. 4

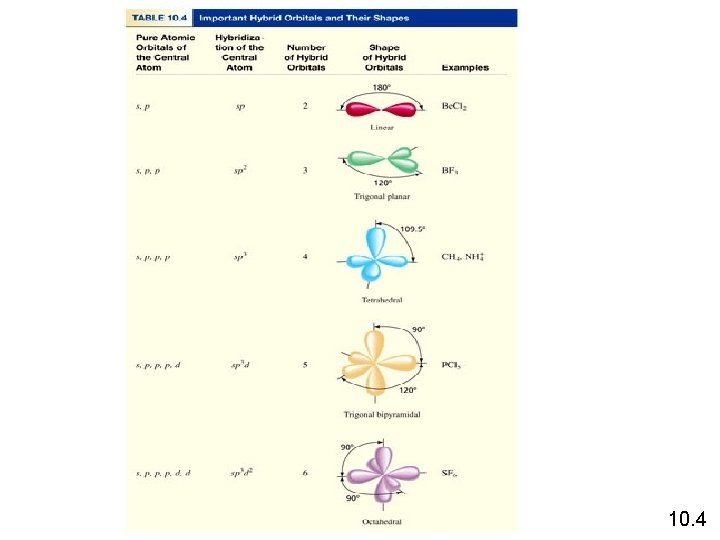
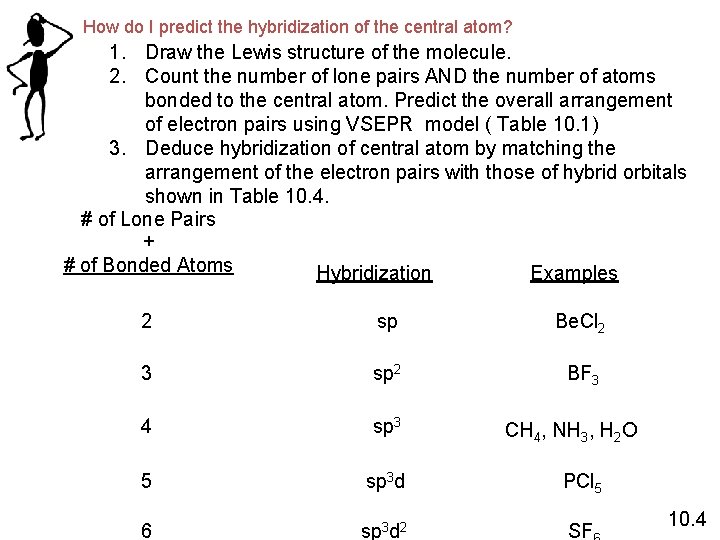
How do I predict the hybridization of the central atom? 1. Draw the Lewis structure of the molecule. 2. Count the number of lone pairs AND the number of atoms bonded to the central atom. Predict the overall arrangement of electron pairs using VSEPR model ( Table 10. 1) 3. Deduce hybridization of central atom by matching the arrangement of the electron pairs with those of hybrid orbitals shown in Table 10. 4. # of Lone Pairs + # of Bonded Atoms Hybridization Examples 2 sp Be. Cl 2 3 sp 2 BF 3 4 sp 3 5 sp 3 d PCl 5 6 sp 3 d 2 SF CH 4, NH 3, H 2 O 10. 4

Molecular Orbital (MO) Configurations -MOs follow the same filling rules as atomic orbitals 1. The number of molecular orbitals (MOs) formed is always equal to the number of atomic orbitals combined. 2. The more stable the bonding MO, the less stable the corresponding antibonding MO. 3. The filling of MOs proceeds from low to high energies. 4. Each MO can accommodate up to two electrons. 5. Use Hund’s rule when adding electrons to MOs of the same energy. (Electrons spread out as much as possible, with spins unpaired, over orbitals that have the same energy) 6. The number of electrons in the MOs is equal to the sum of all the electrons on the bonding atoms. 10. 7

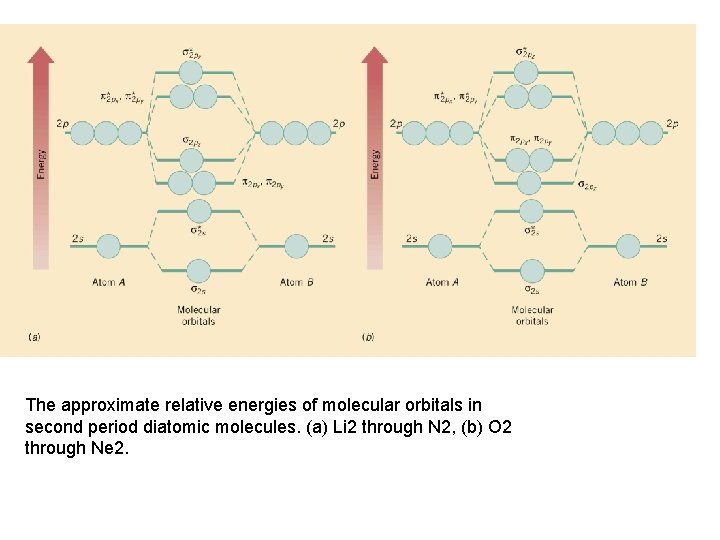
The approximate relative energies of molecular orbitals in second period diatomic molecules. (a) Li 2 through N 2, (b) O 2 through Ne 2.

10. 7

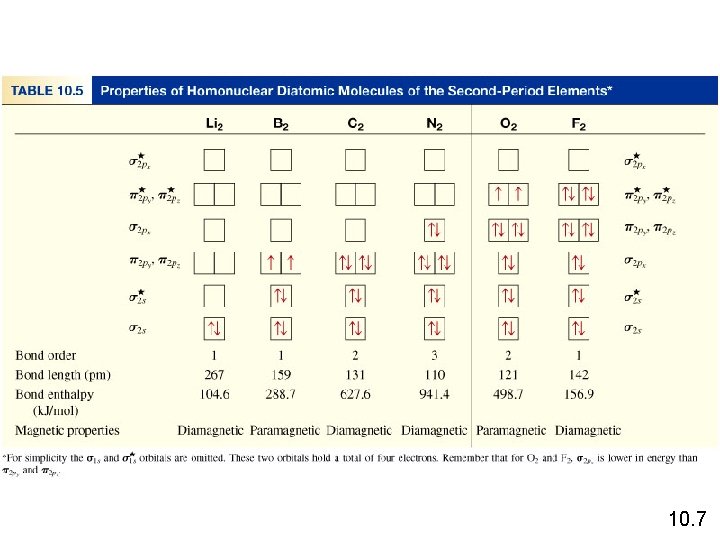
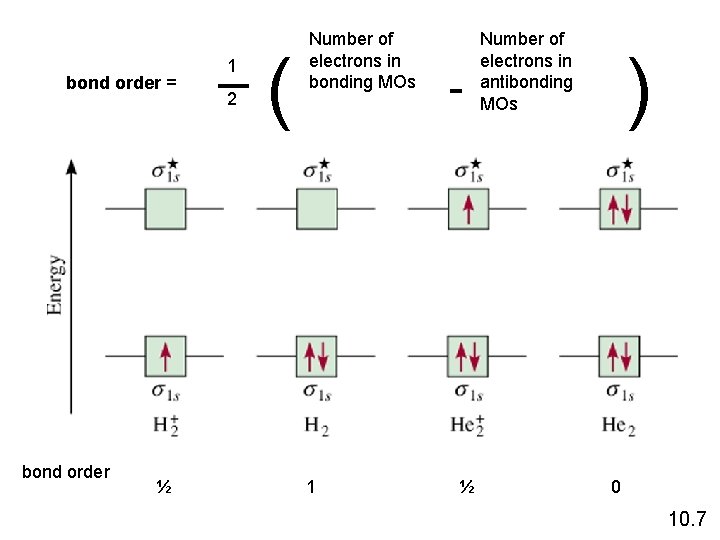
bond order = bond order ½ 1 2 ( Number of electrons in bonding MOs 1 - ½ Number of electrons in antibonding MOs ) 0 10. 7

Chapter 11

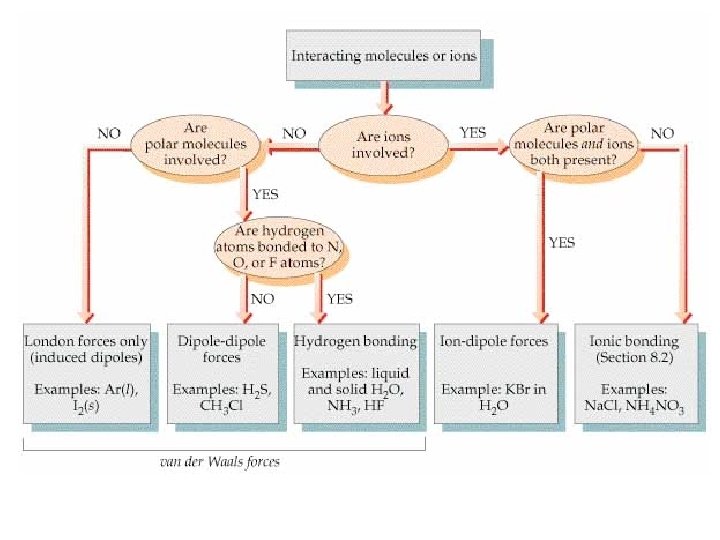
Types of Intermolecular Forces (IMF) • Should actually be called Interparticulate Forces (molecules, ions, and/or atoms) • Ion - ion forces • Ion-dipole forces • Dipole-dipole forces • Dispersion Forces • Hydrogen Bonds Ion - ion forces: (lattice energy-ionic compound) • Remember Chapter 9 • Force depends on the charge on the ions and the distance separating the ions • (STRONG FORCES) E=k Q+ Qr


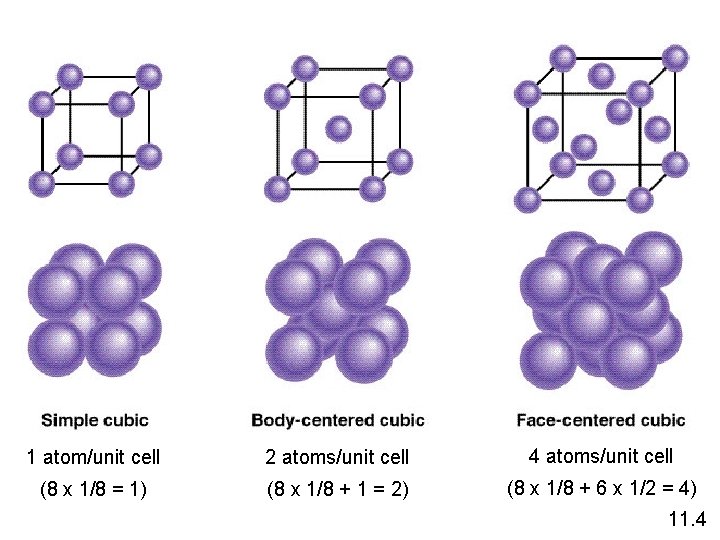
1 atom/unit cell 2 atoms/unit cell 4 atoms/unit cell (8 x 1/8 = 1) (8 x 1/8 + 1 = 2) (8 x 1/8 + 6 x 1/2 = 4) 11. 4

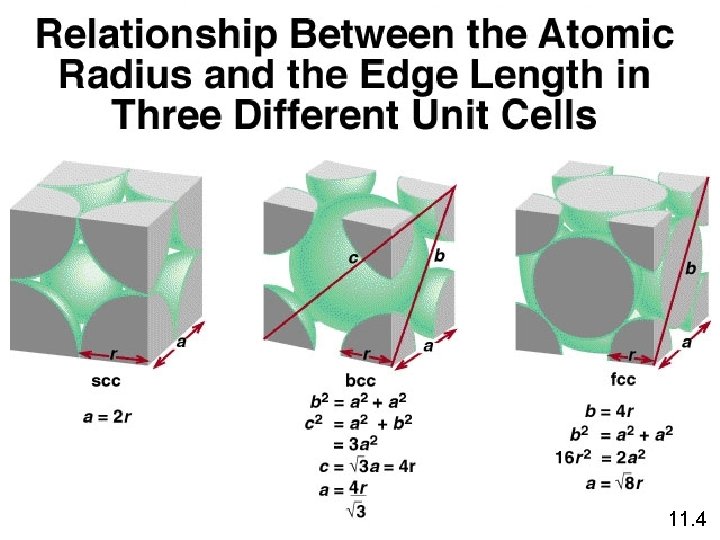
11. 4

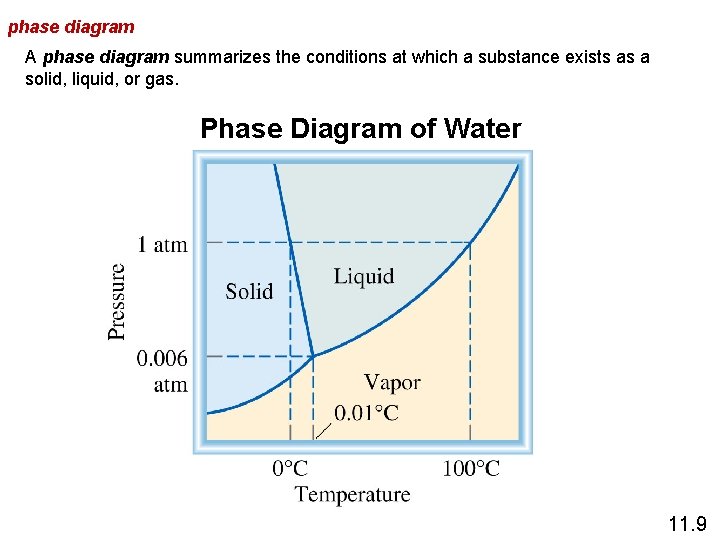
phase diagram A phase diagram summarizes the conditions at which a substance exists as a solid, liquid, or gas. Phase Diagram of Water 11. 9