Density What is density Density compares the mass

- Slides: 13

Density

What is density? • Density compares the mass of an object to its volume

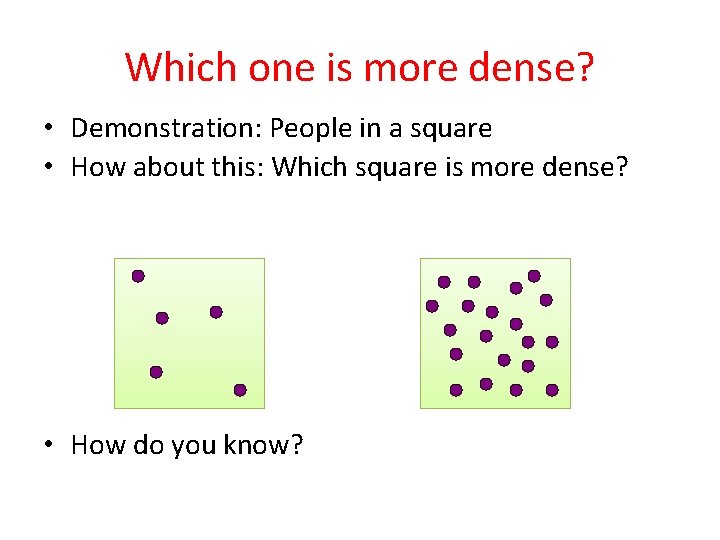
Which one is more dense? • Demonstration: People in a square • How about this: Which square is more dense? • How do you know?

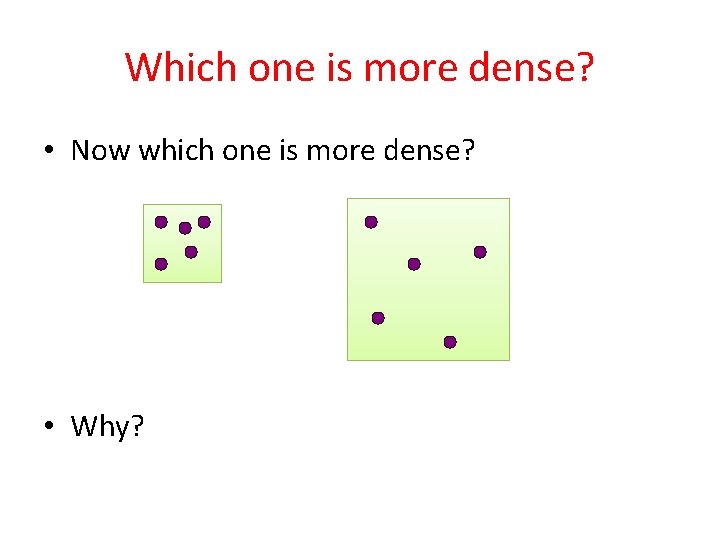
Which one is more dense? • Now which one is more dense? • Why?

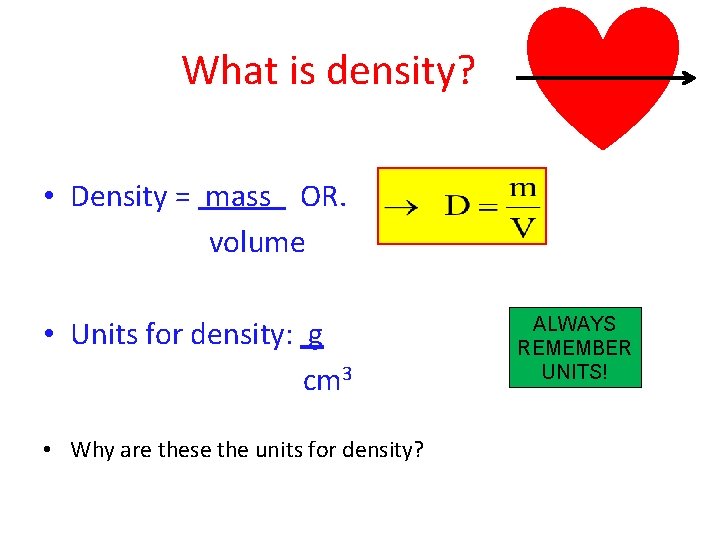
What is density? • Density = mass OR. volume • Units for density: g cm 3 • Why are these the units for density? ALWAYS REMEMBER UNITS!

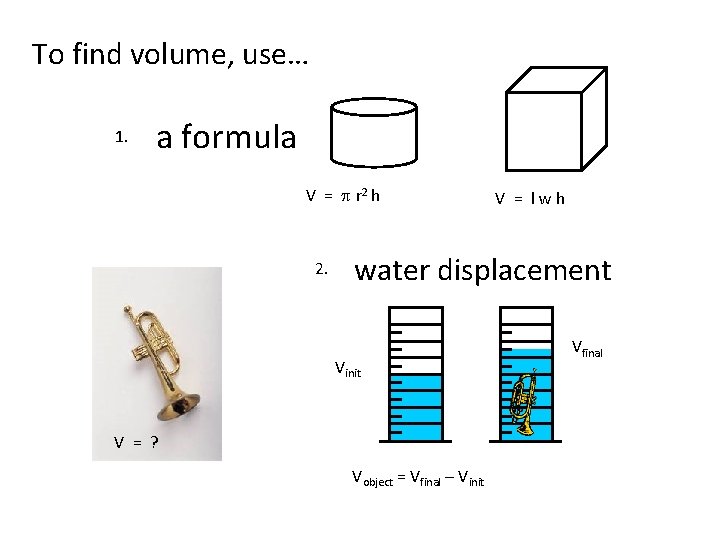
To find volume, use… 1. a formula V = p r 2 h 2. V = lwh water displacement Vinit V = ? Vobject = Vfinal – Vinit Vfinal

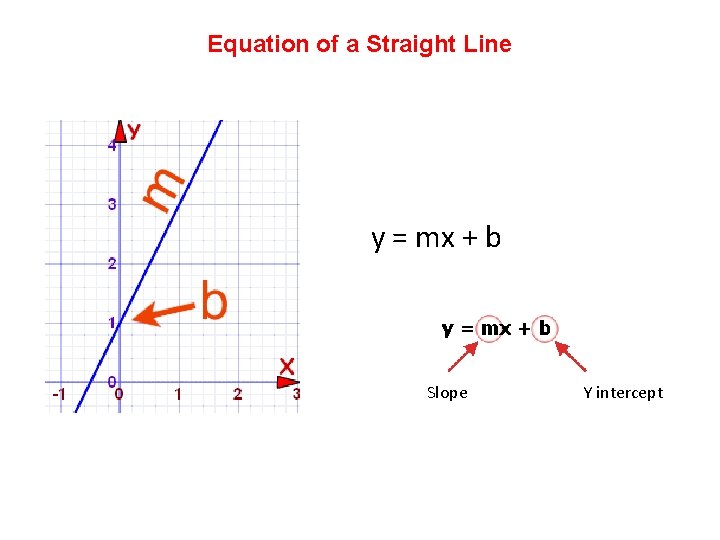
Equation of a Straight Line y = mx + b Slope Y intercept

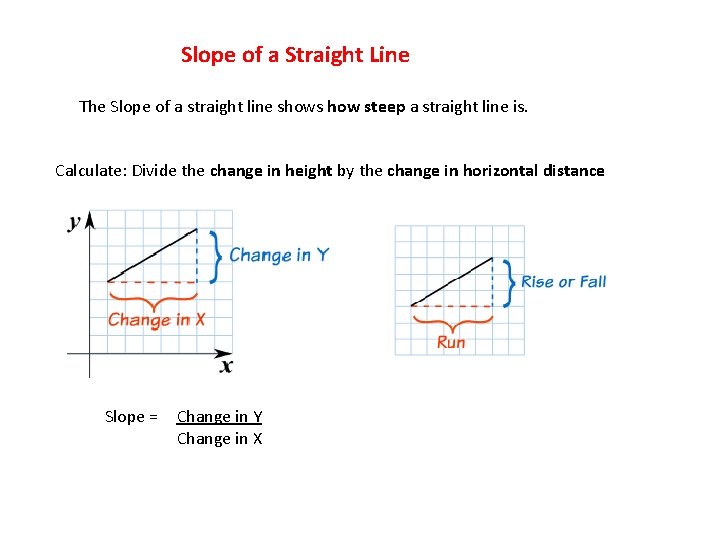
Slope of a Straight Line The Slope of a straight line shows how steep a straight line is. Calculate: Divide the change in height by the change in horizontal distance Slope = Change in Y Change in X

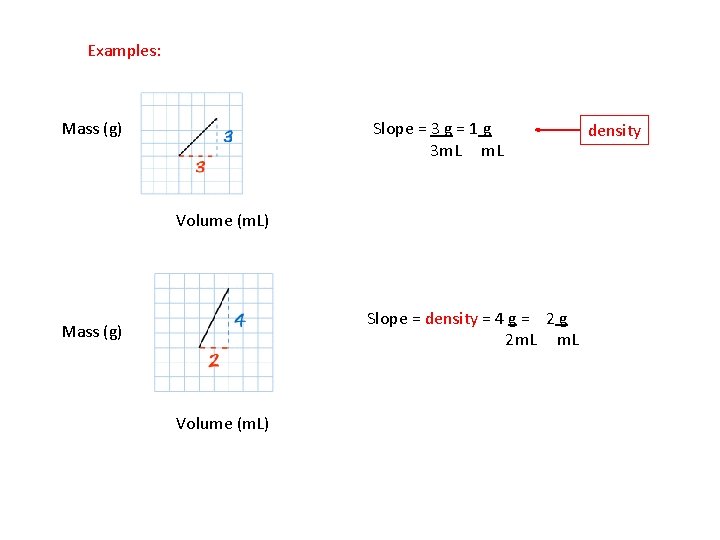
Examples: Mass (g) Slope = 3 g = 1 g 3 m. L Volume (m. L) Slope = density = 4 g = 2 g 2 m. L Mass (g) Volume (m. L) density

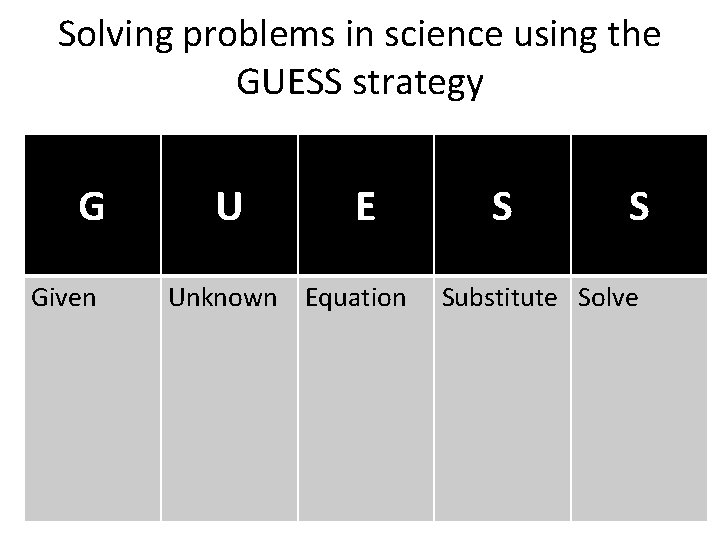
Solving problems in science using the GUESS strategy G Given U E Unknown Equation S S Substitute Solve

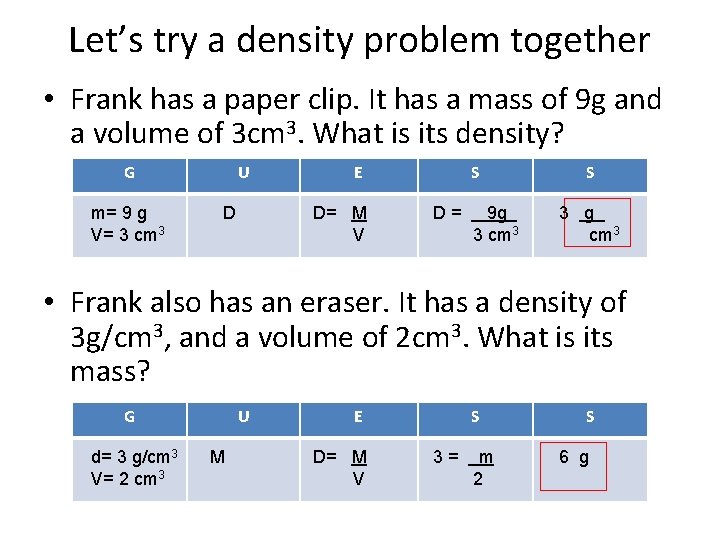
Let’s try a density problem together • Frank has a paper clip. It has a mass of 9 g and a volume of 3 cm 3. What is its density? G m= 9 g V= 3 cm 3 U D E D= M V S D= 9 g 3 cm 3 S 3 g cm 3 • Frank also has an eraser. It has a density of 3 g/cm 3, and a volume of 2 cm 3. What is its mass? G d= 3 g/cm 3 V= 2 cm 3 U M E D= M V S 3= m 2 S 6 g

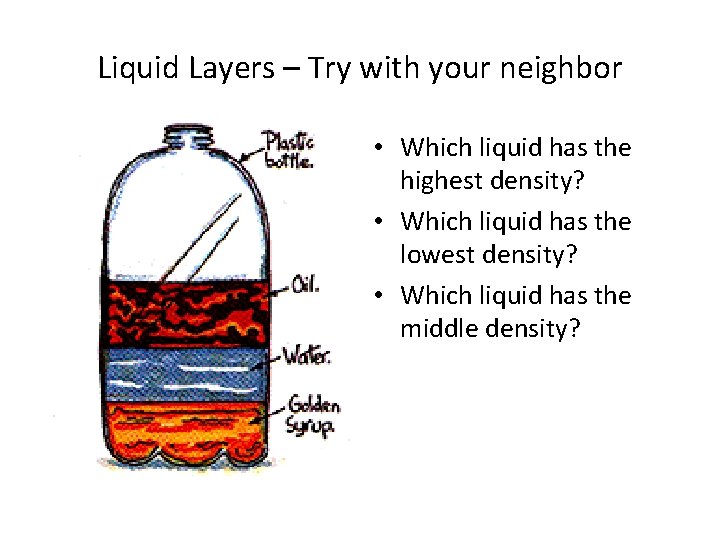
Liquid Layers – Try with your neighbor • Which liquid has the highest density? • Which liquid has the lowest density? • Which liquid has the middle density?

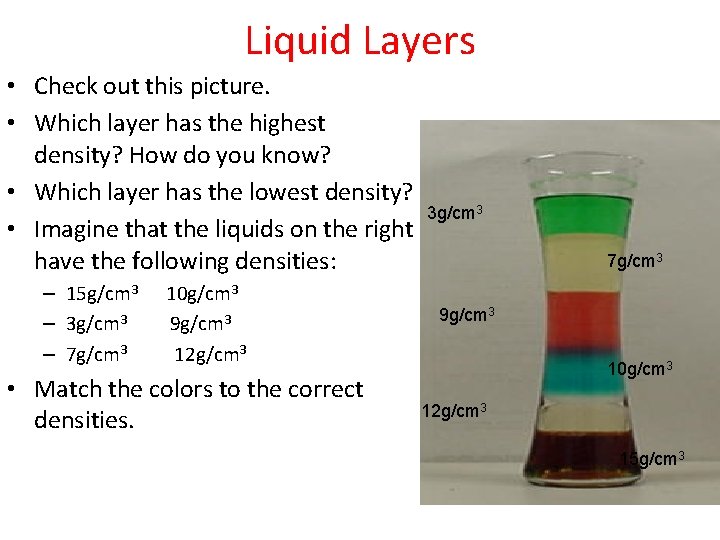
Liquid Layers • Check out this picture. • Which layer has the highest density? How do you know? • Which layer has the lowest density? • Imagine that the liquids on the right have the following densities: – 15 g/cm 3 – 3 g/cm 3 – 7 g/cm 3 10 g/cm 3 9 g/cm 3 12 g/cm 3 • Match the colors to the correct densities. 3 g/cm 3 7 g/cm 3 9 g/cm 3 10 g/cm 3 12 g/cm 3 15 g/cm 3